Published online Nov 24, 2021. doi: 10.5306/wjco.v12.i11.1072
Peer-review started: February 23, 2021
First decision: May 4, 2021
Revised: May 5, 2021
Accepted: October 11, 2021
Article in press: October 11, 2021
Published online: November 24, 2021
Myxopapillary ependymoma (MPE) is a pathological grade I tumor that arises in the filum terminale. MPE with anaplastic features is extremely rare, and only 5 cases have shown malignancy at the time of recurrence.
The patient (a 46-year-old woman) had undergone a MPE operation 30 years ago. After subtotal resection of the tumor located in L4-S1, it had a solid component that extended to the adjacent subcutaneous region. Histologically, the tumor consisted of a typical MPE with anaplastic features. The anaplastic areas of the tumor showed hypercellularity, a rapid mitotic rate, vascular proliferation, and connective tissue proliferation. Pleomorphic cells and atypical mitotic figures were occasionally observed. The MIB-1 index in this area was 12.3%. The im
Although extremely rare, anaplastic MPE occurs in both pediatric and adult patients, similar to other ependymomas. At a minimum, close monitoring is recommended, given concerns about aggressive biological potential. In the future, further study is needed to determine the WHO classification criteria and genetic indicators of tumor progression. The possibility of malignant transformation of MPE should be taken into account, and patients with MPE should be treated with care and follow-up.
Core Tip: Myxopapillary ependymoma (MPE) is a pathological grade I tumor that develops in the filum terminale. MPEs with anaplastic features are extremely rare; only 5 cases have shown malignancy when they relapsed. Here we report a case of MPE with anaplastic features in local late recurrence in a 46-year-old woman and review anaplastic MPE in the published literature. MPEs have the potential for malignant transformation after a long period of time despite being a pathological grade I tumor. Therefore, the possibility of malignant transformation of the MPE should be considered, and patients with MPE should be treated carefully and monitored over a long period of time.
- Citation: Kanno H, Kanetsuna Y, Shinonaga M. Anaplastic myxopapillary ependymoma: A case report and review of literature. World J Clin Oncol 2021; 12(11): 1072-1082
- URL: https://www.wjgnet.com/2218-4333/full/v12/i11/1072.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i11.1072
Myxopapillary ependymomas (MPEs, grade I) account for 9%-13% of ependymal tumors and about 83% of ependymomas are found in the area of the filum terminale[1]. MPEs are typically encapsulated, slow-growing benign neuroepithelial tumors that occur primarily in regions of the medullary conus and filum terminale, and removal without breaking the capsule is curative[2,3]. The average age at presentation is approximately 36 years, with a significant male prediction[1]. They differ morphologically and biologically from other ependymomas and often require immunohistochemical analysis to distinguish them from phenotypically similar tumors[4]. Despite an overall favorable prognosis and classification as a grade I tumor, MPEs have been associated with distant metastases, subarachnoid disseminations and local late recurrences[5-8].
In the 2016 WHO classification of central nervous system tumors, ependymal tumors are classified into the following five subtypes: MPE and subependymoma, and MPE (grade I), classic ependymoma (grade II), RELA fusion protein positive ependymoma (grade II / III) and anaplastic ependymoma (grade III)[1]. MPEs account for 9%-13% of ependymal tumors and around 83% of ependymomas are found in the area of the filum terminale[2]. MPEs are usually benign, slow growing neuroepithelial tumors that occur predominantly as intradural neoplasms in the region of the medullary conus, cauda equine, and filum terminale, although rare occurrences have been reported in the neck, thoracic spinal cord, lateral ventricles, and cerebral parenchyma[3-6,9]. MPEs are usually encapsulated and removal without damaging the capsule is curative[2,3]. Distant metastases from MEPs in the brain parenchyma and other organs have also been reported[10-20]. They differ morphologically and biologically from other ependymomas and immunohistochemical study is often required for differential diagnosis from chordomas or chondrosarcomas that morphologically resemble ependymomas[6]. The mean age at manifestation is around 36 years, with a male predominance[1]. Anaplastic features in glial tumors including ependymomas include frequent mitotic figures, hypercellularity, necrosis, vascular proliferation, and pleomorphologic cytoplasm and nuclei[21]. However, classification and grading of ependymomas with anaplastic features are historically controversial. Hence, their diagnosis is difficult and subjective. MPEs with anaplastic features are usually locally invasive. They frequently tend to disseminate to other areas of the brain and spinal cord via cerebrospinal fluid (CSF), and more frequently recur with a shorter survival[1,21]. Grade II gliomas often transform into more malignant types[22]. However, grade I gliomas rarely transform into more malignant types[23], and MPEs have only shown malignant transformation in 4 cases[24]. MPEs with anaplastic features are extremely rare in the literature (20 cases)[25-29] and recurrent MPEs have only shown malignant transformation in 5 cases[29]. We report the case of a 46-year-old woman with MPE with anaplastic features and local ultra-late recurrence. Clinical and histopathological findings are described and the malignant transformation of the MPE is discussed.
Lumbar to sacral pain and weak legs for 6 mo.
The patient (a 46-year-old woman) had lumbar to sacral pain and leg weakness for 6 mo and attended the Atami Hospital of the International University of Health and Welfare.
The patient underwent a subtotal resection for a myxopapillary filum ependymoma 30 years ago by the same surgeon.
No other relevant personal or family history.
The neurological examination showed bilateral muscle weakness of the gastrocnemius, anterior tibia and urethral sphincter as well as sensory disturbances in areas of L4, L5 and S1.
Laboratory examinations showed normal levels of all parameters tested.
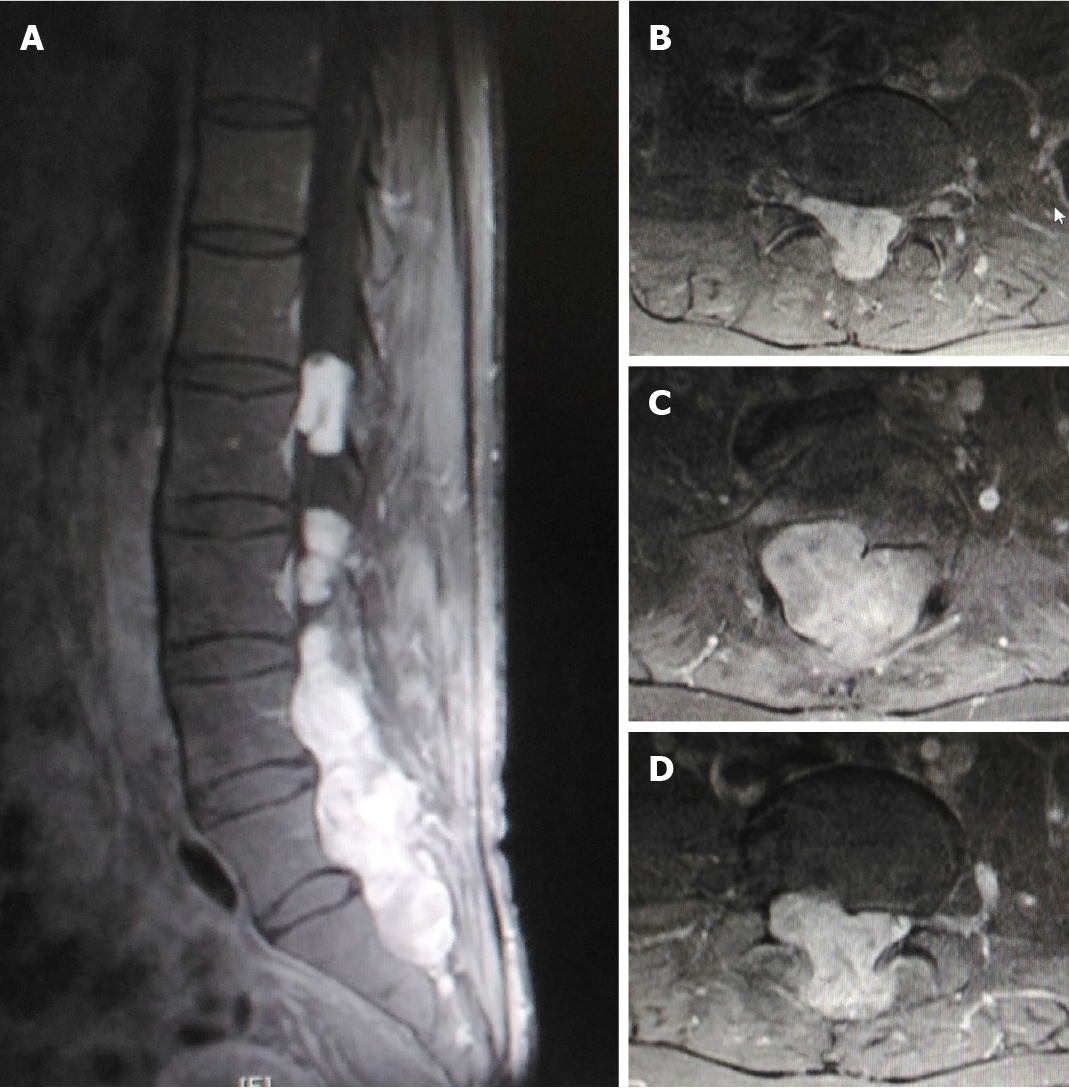
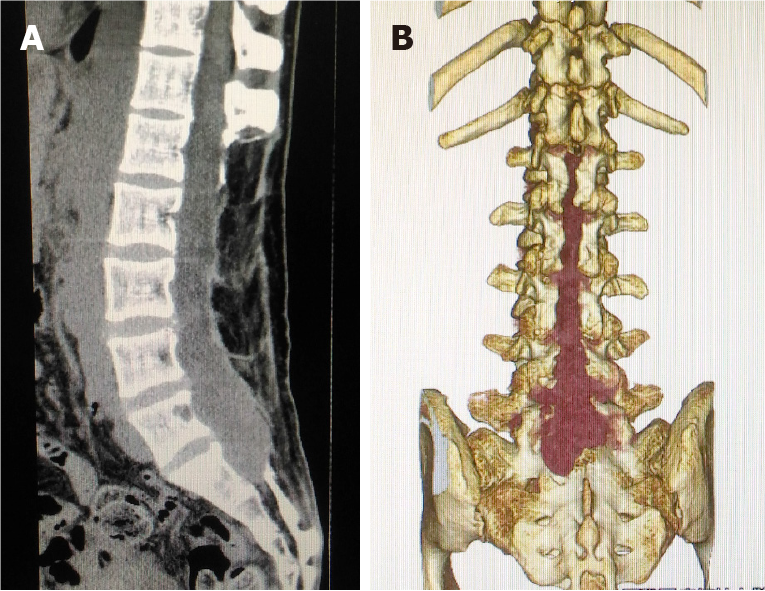
Magnetic resonance imaging (MRI) (Figure 1) and computed tomography (CT) imaging (Figure 2) studies showed a mass that occupied most of the spinal canal from L2 to S1 and extended into the adjacent subcutaneous region.
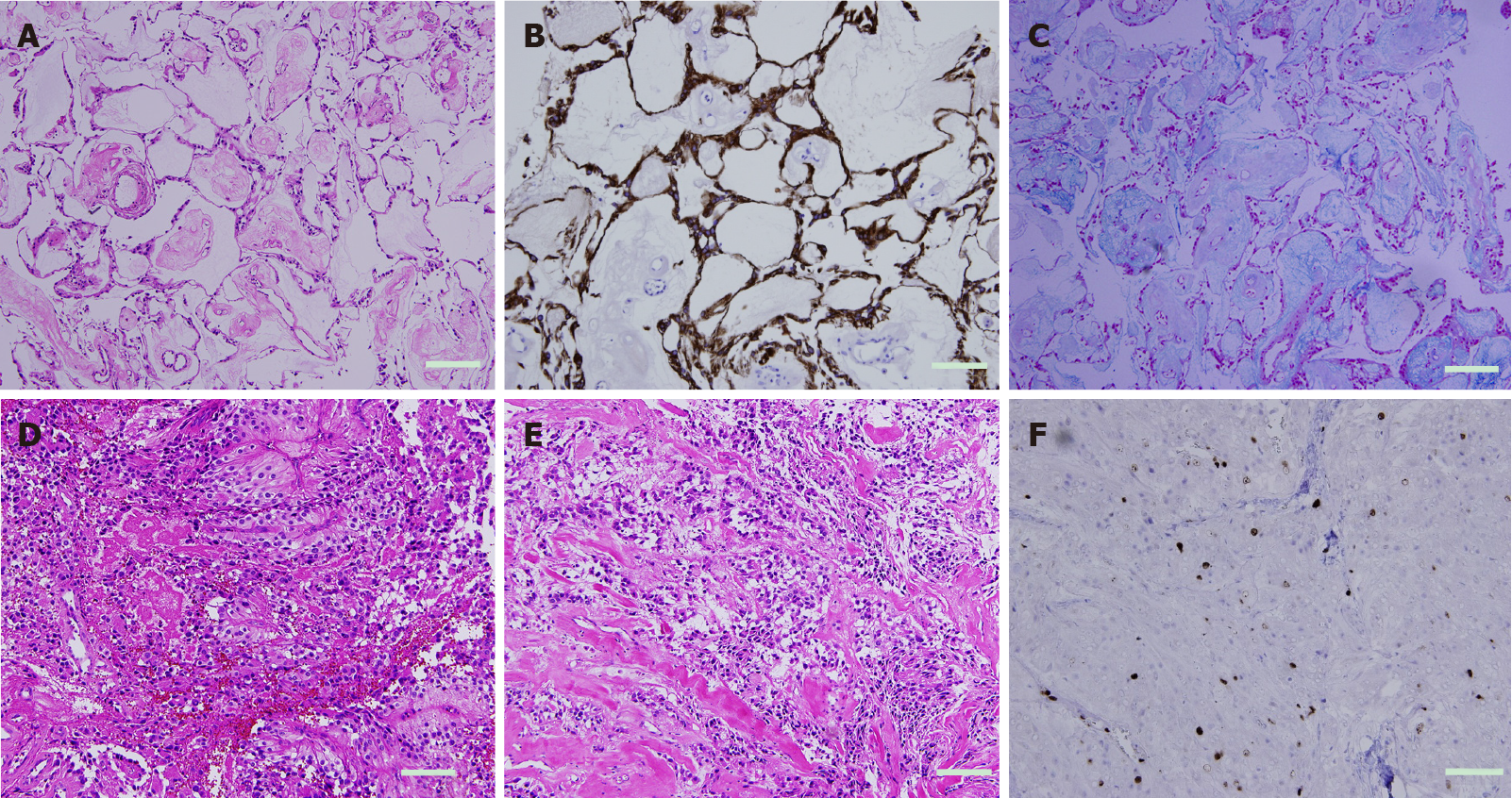
Examination of the tumor with hematoxylin and eosin staining showed neoplastic cells with round nuclei and clear cytoplasm in the background of the fibrillar stroma. These neoplastic cells formed two transition structures: A typical MPE area and a high quality area with anaplastic features. The low-grade area of the tumor had the following appearance: Typical MPE area with a somewhat poor and pointed arrangement with Alcian blue-positive myxoid matrix. The MIB-1 index in this area was 5.1%. On the other hand, the high-grade anaplastic areas of the tumor showed hypercellularity, a rapid mitotic rate, vascular proliferation, and connective tissue proliferation. Pleomorphic cells and atypical mitotic figures were occasionally seen. The MIB-1 index was 12.3%. The immunohistochemical study showed immunoreactivity for vimentin, glial fibrillary acidic protein (GFAP) and S100, but no immunoreactivity for epithelial membrane antigen and chromogranin A (Figure 3). The morphological pattern and immunohistochemical profile were consistent with an MPE with anaplastic features[29]. The combination of areas of typical MPE-appearing tumor interspersed with areas of ependymoma with anaplastic features was consistent with the diagnosis of MPE with anaplastic features. The final diagnosis was MPE with anaplastic features (Figure 4).
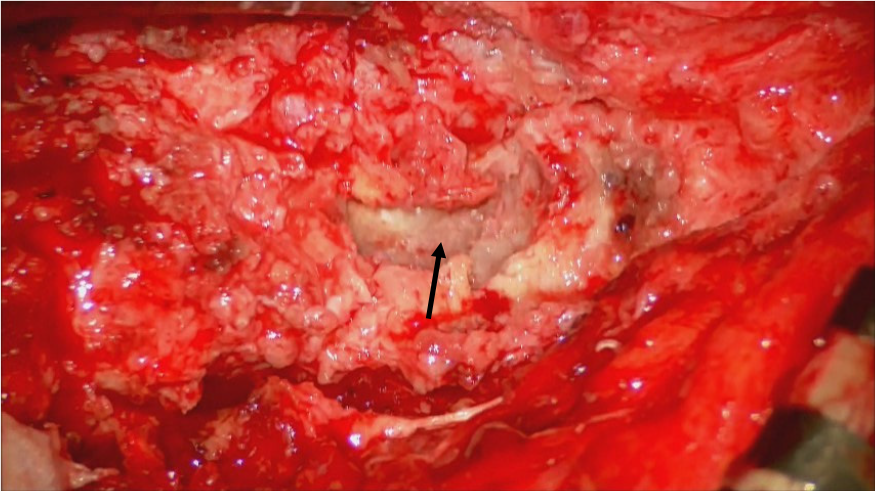
Prior to surgical treatment, written informed consent was obtained from the patient. After placing the patient in the prone position, a midline incision was made at 15 cm from L2 to S2. After exposing the rostral end of the previous laminectomy at L2, the previous laminectomy area up to S2 was exposed. In addition, the lamina sacralis was partially drilled. The dura mater was adhered to the granulated soft tissue and opened, but no CSF was found. The encapsulated tumor was observed (Figure 3). The tumor was dark red mixed with gray and appeared to be highly fibrous and hemorrhagic. The filum terminale was compressed by the tumor at the L2 level. Part of the filum penetrated the tumor and was scarified by fibrous restriction due to the difficult dissection. The tumor was debulked with a Cavitron ultrasonic surgical aspirator (Integra Life Science, Dublin, Ireland) and a subtotal resection was performed. Postoperatively, the patient was well without any new neurological deficits.
Postoperatively, the patient showed no new neurological deficits; and bladder and bowel functions were intact. She presented unchanged motor strength in the lower extremities. She had local radiation of the residual tumor and rehabilitation. She was examined again and showed no deterioration.
According to a recent report[24], anaplasia of MPE was defined on the basis of histopathological findings that are similar to the criteria currently used to define anaplasia in classical ependymomas, that is, they have at least two of the following features: Mitosis per 10 high power field (HPF), MIB-1 labeling index > 10%, microvascular proliferation and spontaneous necrosis. In the present case, the MIB-1 index of 12.3% corresponds to previous reports. In addition, endothelial proliferation was found in the present case. These pathological findings are compatible with anaplastic MPE. The immunohistochemical description of MPE has been reported, which, in agreement with the present case, consists of a positivity for GFAP and vimentin[16,30,31]. In the present case, interestingly, the histopathological features showed anaplastic features mixed with those of three different components of low-grade ependymoma including MPE. This finding could suggest that an original MPE cell has the potential to become different types of ependymomas that are molecularly different[32]. Recent genetic analyses have shown that MPE is characterized by genome-wide polyploidy, often between several chromosomes[33]. MPE shows specific losses of chr16 and chr12 and increases of chr4, chr9 and chr18, while the classical grade II ependymoma shows a specific loss of chr16 and an increase of chr12[34]. MPE differs molecularly, transcriptionally and histologically from classical 2nd degree ependymoma. Gene expression profiling also showed that MPEs have a Warburg phenotype[33] and increased gene expression of HOXB13 compared to non-MPEs[34]. The specific familial, epigenetic, or environmental cause that predisposes to malignant transformation of MPE has not been identified. In contrast to grade II gliomas, which have a high progression to high-grade gliomas, grade I gliomas such as pilocytic astrocytoma, ganglioglioma, and MPE rarely undergo malignant transformation with a maximum incidence of 10%[22,23]. Malignant transformation occurred spontaneously or after radiation therapy in these tumors. The specific familial, epigenetic, or environmental cause that predisposes to malignant transformation of the MPE has not been identified.
Similar to previous reports, the present case showed a large mass occupying the spinal canal. In spite of an overall favorable prognosis and classification as a grade I tumor, MPEs have been associated with distant metastases, subarachnoid disseminations, and local late recurrences in almost half of all patients, regardless of adequate resection[3,4,6,7,35-40]. In 20 reported cases of anaplastic MPE, including this case, the age of the patients ranged from 0.9 to 57 years, with a mean of 24.7 years. The majority of patients with anaplastic MPE were under 20 years old, while in a study of 183 patients with classic MPE the mean age at diagnosis was 35.5 ± 15.8 years. CSF dissemination and involvement of adjacent tissue were observed in 50% and 50% of anaplastic MPEs, while distant metastases to the spinal cord and brain were observed in 9.3% and 6% of anaplastic MPEs, respectively[6]. Other studies on classical MPEs have reported higher rates of CSF dissemination of 35% to 57%[7,41], particularly in pediatric cases of MPE and including pediatric dissemination at first presentation in 14%-58% of pediatric cases[8,41]. In the present case, aggressive clinical features such as soft tissue invasion were identified, while distant metastases were not observed. Therefore, many of these patients, particularly those with aggressive clinical features, can undergo radiation therapy. Frequent surveillance scans of patients with MPE are recommended for early detection of recurrent disease with anaplastic features. The 5-year survival rate of patients with non-MPE anaplastic ependymoma has been reported to be less than 20%. The classification of ependymal tumors is currently difficult and according to the current WHO guidelines of questionable clinical benefit[9].
The histopathological criteria for anaplastic ependymoma are as follows: Mitoses (> 4/10 per HPF), hypercellularity, endothelial proliferation, and necrosis[42]. In addition, it has been suggested that anaplastic ependymoma could be defined by the presence of two of these parameters[42]. Large retrospective series of MPE have been conducted, including a large multi-institutional series with 183 patients[6] who had a 10-year overall survival (OS) of 92.4% and a 5- and 10-year progression-free survival (PFS) of 69.5% and 61.2%, respectively. Local MPE relapse occurred in 84% of patients and leptomeningeal spread was observed in 9.3% of patients.
A strong correlation was found between surgical capsule injury and recurrence[43]. A 10-year OS of over 90% was recently confirmed in an epidemiology, surveillance, and outcome analysis of 773 MPE patients[44]. Presacral MPE showed a worse prognosis than MPE in the filum terminale/cauda equina.
According to the 2017 EANO guideline for ependymal tumors, with the exception of gross-total removal (GTR), no factors have been defined that influence the prognosis of spinal cord ependymomas other than MPE[45]. Advances in microsurgical techniques have enabled en bloc GTR, which is the standard treatment for spinal cord ependymoma. GTR mostly resulted in good functional outcomes, and a good functional result was related to small tumor size and little neurological deficit at surgery. Therefore, surgery at an early stage should be considered[46,47]. If GTR cannot be performed due to infiltration of the spinal cord or nerve roots, postoperative local radiation therapy is often used. Multivariate analysis showed that the tumor grade and the extent of resection were independent prognostic factors for OS and PFS, and that radiation therapy extended PFS in patients undergoing subtotal removal (STR). Studies suggest that doses > 50 Gy lead to either better or equivalent results[48,51]. With respect to conventional chemotherapy, it is reported that continuous oral etoposide for recurrent intramedullary ependymomas is well tolerated[53]. Bevacizumab may be of clinical benefit in some patients[52]. Large retrospective series of MPE suggested that the extent of resection was an important independent factor in forecasting local control, while younger age (< 36 years) was a negative prognostic factor. On the other hand, the irregular shape surrounding nerve roots and the formation of a myxoid matrix are related to the risk of postoperative neurological disability[43]. In the treatment of MPE, it has been shown that postoperative radiation therapy with high doses (≥ 50 Gy), compared to patients treated with only one procedure, leads to better local control and longer PFS without significant late toxicity[51,52]. In a small series of adult patients with spinal MPE, it was shown that patients treated with GTR followed by adjuvant radiation therapy had better local control than patients treated with GTR alone[3]. Although patients often have a disseminated tumor and/or develop recurrent or progressive disease after treatment[54], OS is estimated to be 97% and 95% after 5 and 10 years, respectively[55]. A recent series from the Johns Hopkins Hospital[56] showed a significant reduction in local failure in patients receiving radiation therapy after STR or GTR. A smaller series[57] also confirmed good local control with surgery and radiation therapy compared to GTR alone. In summary, according to the 2017 EANO guideline for ependymal tumors[45], the following key recommendations for the treatment of WHO grade I and anaplastic (WHO grades II and III) MPEs are proposed: Total resection is the goal of MPE-surgery (II B). MRI after surgery should be performed to assess the extent of the resection (N/A). Since all patients with newly diagnosed ependymoma are at risk of CSF dissemination, disease staging, including craniospinal MRI and CSF cytology, is recommended after surgery (N/A). A watch-and-wait strategy is recommended for WHO grade II ependymomas after total resection (IIIC). In the case of anaplastic (WHO grade II and III) MPE, postoperative radiation therapy with doses of 45-54 Gy is recommended, irrespective of the extent of the resection (IIIC). Following the incomplete resection of an MPE of WHO grade I, postoperative local radiation therapy with a dose of 50 Gy is recommended (IIB). In the event of relapse, reoperation, re-radiation, and chemotherapy should be considered (IIIC). As the risk of later relapse exists, patients should be followed up with an enhanced MRI over a long period (N/A).
Anaplastic MPE is an extremely rare event and 19 cases have been reported in the literature[24-29]. In 20 reported cases of anaplastic MPE, including this case, the patients ranged in age from 0.9 years to 57 years, with a mean of 24.7 years. The majority of patients with anaplastic MPE were under 20 years old, while in a study of 183 patients with classic MPE the mean age at diagnosis was 35.5 ± 15.8 years. Of the previously reported MPEs with anaplastic features, anaplasia was present in only 5 cases with only one recurrence. In addition, the present case is the most recent relapse in the literature. Similar to previous reports, the present case showed a large mass occupying the spinal canal, and aggressive clinical features such as soft tissue invasion were identified, while distant metastases and CSF dissemination were not observed (Table 1). Despite being histological grade 1 tumors, typical MPEs are often associated with distant metastases, CSF disseminations and local late recurrences. Therefore, patients with a typical MPE can be irradiated after surgery, and it is recommended that patients with MPE be monitored regularly to detect distant metastases or local recurrences[1,2]. In the present case, the patient was not irradiated after the previous operation 30 years ago due to the lack of histological anaplasia. If the patient had been irradiated after the previous operation, recurrence could have been avoided. A 5-year survival rate of less than 20% has been reported for anaplastic ependymoma without MPE. The histopathological parameters of anaplastic ependymoma include > 4 mitoses per 10 HPF, endothelial proliferation, hypercellularity, and necrosis, and the presence of two of these parameters define the diagnosis of anaplastic ependymoma[42]. However, the classification of ependymomas with pathologically anaplastic features remains controversial. Hence, diagnosing these tumors is difficult and subjective at times. Anaplastic ependymomas often recur and survival time is reduced[2]. In addition, the tumors often spread to other regions of the central nervous system through CSF and are usually locally invasive. Recently, it was suggested that the diagnostic criteria for anaplastic MPE should be based on histopathological findings that include at least two of the following parameters: > 5 mitoses per 10 HPF, > 10% MIB-1 labeling index, spontaneous necrosis, and, microvascular proliferation. In the present case, the MIB-1 index of 12.3% is compatible with previous reports. In addition, endothelial proliferation was found in the present case. These pathological findings were consistent with anaplastic MPE[32]. Malignant transformation of MPE can occur in both pediatric and adult patients and is associated with either relapse, local invasion, CSF spread, or metastatic disease. These anaplastic clinical features indicate a more aggressive biological potential than classic MPE. Therefore, regular close observation is recommended. Further studies are needed to refine the proposed assessment criteria for anaplastic MPE and to identify the genetic biomarkers of tumorigenesis and malignant transformation of MPE[33].
| Ref. | Age (yr) at MPE with anaplasia (age at typical MPE) | Sex | Location of MPE with anaplasia | Adjacent tissues involved | CSF diss | MIB-1 Index | MVP | Treatment | |
| Initial | Recurrence | ||||||||
| Awaya et al[25], 2003 | 15 | M | Th12-L2 | No | No | 10% | Yes | GTR | No |
| Beschorner et al[26], 2007 | 3 | M | Subcutaneous sacrococcyx | Yes | No | 40% | Yes | GTR | No |
| Vega-Orozco et al[27], 2011 | 38 (22) | M | Inguinal node metastasis | Yes | Yes | NA | Yes | STR, RT | RT |
| Chakraborti et al[28], 2012 | 0.9 | F | Subcutaneous sacrococcyx | Yes | No | 70% | Yes | GTR, CT | No |
| Huynh et al[29], 2018 | 24 | F | L2-3 | Yes | Yes | 8%-38% | Yes | GTR | GTR |
| Lee et al[24], 2019 | 6 | F | L4-S1 | No | No | 20% | Yes | GTR, RT | No |
| Lee et al[24], 2019 | 7 | F | T12-L3 | No | No | 11% | Yes | STR, RT | No |
| Lee et al[24], 2019 | 10 | M | L1-2 | Yes | No | 34% | Yes | GTR, RT | No |
| Lee et al[24], 2019 | 10 | M | S1-2 | No | No | 15% | Yes | GTR, RT | No |
| Lee et al[24], 2019 | 11 | M | L4-S3 | No | No | 14% | Yes | GTR | No |
| Lee et al[24], 2019 | 12 | M | Lumbo-sacral | Yes | Yes | 10% | Yes | GTR | GTR, RT |
| Lee et al[24], 2019 | 13 | M | L1-2 | No | Yes | 8% | Yes | STR, RT | No |
| Lee et al[24], 2019 | 20 (16) | F | L3-S1 | No | Yes | 10% | No | GTR | RT |
| Lee et al[24], 2019 | 32 (31) | M | S1 | No | Yes | 10% | Yes | STR, CT, RT | RT |
| Lee et al[24], 2019 | 40 | F | 4th ventricle | No | No | 20% | Yes | STR, RT | No |
| Lee et al[24], 2019 | 45 (31) | F | Extraspinal pelvic | Yes | Yes | 40% | Yes | STR, RT | GTR, CT |
| Lee et al[24], 2019 | 50 | M | L5-S3 | Yes | Yes | 10% | Yes | GTR, RT | No |
| Lee et al[24], 2019 | 55 | F | L1-2 | No | Yes | 20% | Yes | GTR, RT | No |
| Lee et al[24], 2019 | 57 (45) | M | T8-L5 | Yes | Yes | 26% | Yes | STR, RT | GTR, RT |
| Kanno et al, 2021 | 46 (16) | F | L4-S1 | Yes | No | 12% | Yes | STR | STR, RT |
MPE has the potential for malignant transformation after a long period of time despite being a pathological grade I tumor. Therefore, the possibility of malignant transformation of MPE should be considered, and patients with MPE should be carefully managed and followed up over a long period of time.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Muhammed A S-Editor: Wu YXJ L-Editor: Webster JR P-Editor: Wu YXJ
| 1. | McLendon R, Schiffer D, Rosenblum M, Wiestler O. Myxopapillary ependymoma. In: Louis D, H Ohgaki H, Wiestler O, Cavenii W eds. WHO Classification of Tumours of the Central Nervous System, 4th edition, Lyon. France: IARC, 2016: 104-105. [Cited in This Article: ] |
| 2. | Gilbert MR, Ruda R. Ependymal tumours. In: Batchelor TT, Nishikawa R, Tarbell NJ, Weller M. edited Oxford Textbook of Neuro-Oncology. United Kingdom: Oxford University, 2017: 49-56. [Cited in This Article: ] |
| 3. | Akyurek S, Chang EL, Yu TK, Little D, Allen PK, McCutcheon I, Mahajan A, Maor MH, Woo SY. Spinal myxopapillary ependymoma outcomes in patients treated with surgery and radiotherapy at M.D. Anderson Cancer Center. J Neurooncol. 2006;80:177-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Sonneland PR, Scheithauer BW, Onofrio BM. Myxopapillary ependymoma. A clinicopathologic and immunocytochemical study of 77 cases. Cancer. 1985;56:883-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 5. | Pica A, Miller R, Villà S, Kadish SP, Anacak Y, Abusaris H, Ozyigit G, Baumert BG, Zaucha R, Haller G, Weber DC. The results of surgery, with or without radiotherapy, for primary spinal myxopapillary ependymoma: a retrospective study from the rare cancer network. Int J Radiat Oncol Biol Phys. 2009;74:1114-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Weber DC, Wang Y, Miller R, Villà S, Zaucha R, Pica A, Poortmans P, Anacak Y, Ozygit G, Baumert B, Haller G, Preusser M, Li J. Long-term outcome of patients with spinal myxopapillary ependymoma: treatment results from the MD Anderson Cancer Center and institutions from the Rare Cancer Network. Neuro Oncol. 2015;17:588-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Fassett DR, Pingree J, Kestle JR. The high incidence of tumor dissemination in myxopapillary ependymoma in pediatric patients. Report of five cases and review of the literature. J Neurosurg. 2005;102:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Kraetzig T, McLaughlin L, Bilsky MH, Laufer I. Metastases of spinal myxopapillary ependymoma: unique characteristics and clinical management. J Neurosurg Spine. 2018;28:201-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10993] [Cited by in F6Publishing: 9851] [Article Influence: 1231.4] [Reference Citation Analysis (0)] |
| 10. | Lim SC, Jang SJ. Myxopapillary ependymoma of the fourth ventricle. Clin Neurol Neurosurg. 2006;108:211-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Davis C, Barnard RO. Malignant behavior of myxopapillary ependymoma. Report of three cases. J Neurosurg. 1985;62:925-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Kittel K, Gjuric M, Niedobitek G. [Metastasis of a spinal myxopapillary ependymoma to the inner auditory canal]. HNO. 2001;49:298-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Libório R, Pais RF, Soares GB, Rocha A, Ferreira F, Garcia T, Pais FF. [Medullary and intracranial metastases of myxopapillary ependymoma]. Acta Med Port. 2001;14:133-138. [PubMed] [Cited in This Article: ] |
| 14. | Mavroudis C, Townsend JJ, Wilson CB. A metastasizing ependymoma of the cauda equina. Case report. J Neurosurg. 1977;47:771-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Miralbell R, Louis DN, O'Keeffe D, Rosenberg AE, Suit HD. Metastatic ependymoma of the sacrum. Cancer. 1990;65:2353-2355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 16. | Straus D, Tan LA, Takagi I, O'Toole JE. Disseminated spinal myxopapillary ependymoma in an adult at initial presentation: a case report and review of the literature. Br J Neurosurg. 2014;28:691-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Toktaş ZO, Demir MK, Yapıcıer Ö, Akakın A, Yılmaz B, Konya D. Disseminated adult spinal extramedullary myxopapillary ependymoma. Spine J. 2015;15:e69-e70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Wight DG, Holley KJ, Finbow JA. Metastasizing ependymoma of the cauda equina. J Clin Pathol. 1973;26:929-935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Woesler B, Moskopp D, Kuchelmeister K, Schul C, Wassmann H. Intracranial metastasis of a spinal myxopapillary ependymoma. A case report. Neurosurg Rev. 1998;21:62-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Yücesoy K, Ozer E, Koyuncuoglu M. Parenchymal brain metastasis of a spinal myxopapillary ependymoma after extradural manipulation. Acta Neurochir (Wien). 2001;143:1071-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Chi JH, Cachola K, Parsa AT. Genetics and molecular biology of intramedullary spinal cord tumors. Neurosurg Clin N Am. 2006;17:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Broniscer A, Baker SJ, West AN, Fraser MM, Proko E, Kocak M, Dalton J, Zambetti GP, Ellison DW, Kun LE, Gajjar A, Gilbertson RJ, Fuller CE. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25:682-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Avinash KS, Thakar S, Aryan S, Ghosal N, Hegde AS. Malignant Transformation of Pediatric Low-grade Gliomas: Report of Two Cases and Review of a Rare Pathological Phenomenon. Neurol India. 2019;67:1100-1106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Lee JC, Sharifai N, Dahiya S, Kleinschmidt-DeMasters BK, Rosenblum MK, Reis GF, Samuel D, Siongco AM, Santi M, Storm PB, Ferris SP, Bollen AW, Pekmezci M, Solomon DA, Tihan T, Perry A. Clinicopathologic features of anaplastic myxopapillary ependymomas. Brain Pathol. 2019;29:75-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Awaya H, Kaneko M, Amatya VJ, Takeshima Y, Oka S, Inai K. Myxopapillary ependymoma with anaplastic features. Pathol Int. 2003;53:700-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Beschorner R, Wehrmann M, Ernemann U, Bonin M, Horber V, Oehl-Jaschkowitz B, Meyermann R, Dufke A. Extradural ependymal tumor with myxopapillary and ependymoblastic differentiation in a case of Schinzel-Giedion syndrome. Acta Neuropathol. 2007;113:339-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Vega-Orozco R, Rembao-Bojórquez D, Salmerón-Mercado M, García-Marquez A, Tena-Suck ML. Inguinal lymph nodal metastasis of myxopapillary ependymoma confirmed by fine-needle aspiration cytology, biopsy, and immunohistochemistry: case report. Diagn Cytopathol. 2011;39:689-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Chakraborti S, Kini H, Pai KG, Upadhyaya V. Sacrococcygeal myxopapillary ependymoma with anaplastic ependymoma component in an infant. J Pediatr Neurosci. 2012;7:218-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Huynh T, Lu C, Drazin D, Lekovie G. Myxopaplillary epenedymoma with anaplastic features: A case report and the literature. Surg Neurol Int. 2018;9:191. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Ang LC, Taylor AR, Bergin D, Kaufmann JC. An immunohistochemical study of papillary tumors in the central nervous system. Cancer. 1990;65:2712-2719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 31. | Coffin CM, Swanson PE, Wick MR, Dehner LP. An immunohistochemical comparison of chordoma with renal cell carcinoma, colorectal adenocarcinoma, and myxopapillary ependymoma: a potential diagnostic dilemma in the diminutive biopsy. Mod Pathol. 1993;6:531-538. [PubMed] [Cited in This Article: ] |
| 32. | Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F, Wani K, Tatevossian R, Punchihewa C, Johann P, Reimand J, Warnatz HJ, Ryzhova M, Mack S, Ramaswamy V, Capper D, Schweizer L, Sieber L, Wittmann A, Huang Z, van Sluis P, Volckmann R, Koster J, Versteeg R, Fults D, Toledano H, Avigad S, Hoffman LM, Donson AM, Foreman N, Hewer E, Zitterbart K, Gilbert M, Armstrong TS, Gupta N, Allen JC, Karajannis MA, Zagzag D, Hasselblatt M, Kulozik AE, Witt O, Collins VP, von Hoff K, Rutkowski S, Pietsch T, Bader G, Yaspo ML, von Deimling A, Lichter P, Taylor MD, Gilbertson R, Ellison DW, Aldape K, Korshunov A, Kool M, Pfister SM. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell. 2015;27:728-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 772] [Cited by in F6Publishing: 753] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 33. | Mack SC, Agnihotri S, Bertrand KC, Wang X, Shih DJ, Witt H, Hill N, Zayne K, Barszczyk M, Ramaswamy V, Remke M, Thompson Y, Ryzhova M, Massimi L, Grajkowska W, Lach B, Gupta N, Weiss WA, Guha A, Hawkins C, Croul S, Rutka JT, Pfister SM, Korshunov A, Pekmezci M, Tihan T, Philips JJ, Jabado N, Zadeh G, Taylor MD. Spinal Myxopapillary Ependymomas Demonstrate a Warburg Phenotype. Clin Cancer Res. 2015;21:3750-3758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Barton VN, Donson AM, Kleinschmidt-DeMasters BK, Birks DK, Handler MH, Foreman NK. Unique molecular characteristics of pediatric myxopapillary ependymoma. Brain Pathol. 2010;20:560-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Mridha AR, Sharma MC, Sarkar C, Garg A, Singh MM, Suri V. Anaplastic ependymoma with cartilaginous and osseous metaplasia: report of a rare case and review of literature. J Neurooncol. 2007;82:75-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Ilhan A, Furtner J, Birner P, Rössler K, Marosi C, Preusser M. Myxopapillary ependymoma with pleuropulmonary metastases and high plasma glial fibrillary acidic protein levels. J Clin Oncol. 2011;29:e756-e757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Higgins GS, Smith C, Summers DM, Statham PX, Erridge SC. Myxopapillary ependymoma with intracranial metastases. Br J Neurosurg. 2005;19:356-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Al-Hussaini M, Herron B. Metastasizing myxopapillary ependymoma. Histopathology. 2005;46:469-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Fegerl G, Marosi C. Stabilization of metastatic myxopapillary ependymoma with sorafenib. Rare Tumors. 2012;4:e42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Feldman WB, Clark AJ, Safaee M, Ames CP, Parsa AT. Tumor control after surgery for spinal myxopapillary ependymomas: distinct outcomes in adults vs children: a systematic review. J Neurosurg Spine. 2013;19:471-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 41. | Kukreja S, Ambekar S, Sin AH, Nanda A. Cumulative survival analysis of patients with spinal myxopapillary ependymomas in the first 2 decades of life. J Neurosurg Pediatr. 2014;13:400-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Ho DM, Hsu CY, Wong TT, Chiang H. A clinicopathologic study of 81 patients with ependymomas and proposal of diagnostic criteria for anaplastic ependymoma. J Neurooncol. 2001;54:77-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Abdulaziz M, Mallory GW, Bydon M, De la Garza Ramos R, Ellis JA, Laack NN, Marsh WR, Krauss WE, Jallo G, Gokaslan ZL, Clarke MJ. Outcomes following myxopapillary ependymoma resection: the importance of capsule integrity. Neurosurg Focus. 2015;39:E8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Bates JE, Choi G, Milano MT. Myxopapillary ependymoma: a SEER analysis of epidemiology and outcomes. J Neurooncol. 2016;129:251-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Rudà R, Reifenberger G, Frappaz D, Pfister SM, Laprie A, Santarius T, Roth P, Tonn JC, Soffietti R, Weller M, Moyal EC. EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro Oncol. 2018;20:445-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 46. | Tobin MK, Geraghty JR, Engelhard HH, Linninger AA, Mehta AI. Intramedullary spinal cord tumors: a review of current and future treatment strategies. Neurosurg Focus. 2015;39:E14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 47. | Eroes CA, Zausinger S, Kreth FW, Goldbrunner R, Tonn JC. Intramedullary low grade astrocytoma and ependymoma. Surgical results and predicting factors for clinical outcome. Acta Neurochir (Wien). 2010;152:611-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Lin Y, Smith ZA, Wong AP, Melkonian S, Harris DA, Lam S. Predictors of survival in patients with spinal ependymoma. Neurol Res. 2015;37:650-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Oh MC, Ivan ME, Sun MZ, Kaur G, Safaee M, Kim JM, Sayegh ET, Aranda D, Parsa AT. Adjuvant radiotherapy delays recurrence following subtotal resection of spinal cord ependymomas. Neuro Oncol. 2013;15:208-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Shaw EG, Evans RG, Scheithauer BW, Ilstrup DM, Earle JD. Radiotherapeutic management of adult intraspinal ependymomas. Int J Radiat Oncol Biol Phys. 1986;12:323-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 97] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Chamberlain MC. Etoposide for recurrent spinal cord ependymoma. Neurology. 2002;58:1310-1311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | The response of spinal cord ependymomas to bevacizumab in patients with neurofibromatosis Type 2. J Neurosurg Spine. 2017;26:474-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Schild SE, Nisi K, Scheithauer BW, Wong WW, Lyons MK, Schomberg PJ, Shaw EG. The results of radiotherapy for ependymomas: the Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 1998;42:953-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 116] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Schild SE, Wong W, Nisi K. In regard to the radiotherapy of myxopapillary ependymomas. Int J Radiat Oncol Biol Phys. 2002;53:787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Lucchesi KM, Grant R, Kahle KT, Marks AM, DiLuna ML. Primary spinal myxopapillary ependymoma in the pediatric population: a study from the Surveillance, Epidemiology, and End Results (SEER) database. J Neurooncol. 2016;130:133-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Agbahiwe HC, Wharam M, Batra S, Cohen K, Terezakis SA. Management of pediatric myxopapillary ependymoma: the role of adjuvant radiation. Int J Radiat Oncol Biol Phys. 2013;85:421-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Al-Halabi H, Montes JL, Atkinson J, Farmer JP, Freeman CR. Adjuvant radiotherapy in the treatment of pediatric myxopapillary ependymomas. Pediatr Blood Cancer. 2010;55:639-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |