Vibrio Species in an Urban Tropical Estuary: Antimicrobial Susceptibility, Interaction with Environmental Parameters, and Possible Public Health Outcomes
Abstract
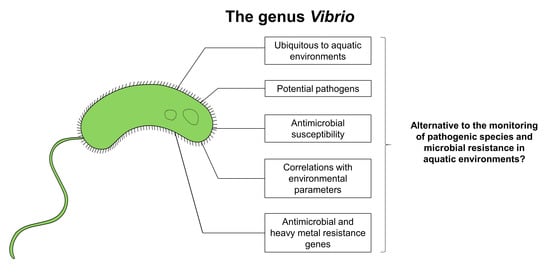
:1. Introduction
2. Materials and Methods
2.1. Water Sampling and Processing
2.2. Vibrio Isolation
2.3. Identification and Characterization of Vibrio
2.4. Antimicrobial Susceptibility Testing
2.5. Phenotypic Detection of Extended-Spectrum Beta-Lactamase Producers
2.6. Detection of Beta-Lactam and Heavy Metal Resistance Genes, and Virulence Genes
2.7. Data Analysis and Statistics
2.8. BOX-PCR
3. Results
3.1. Bacterial Isolation, Characterization, and Identification
3.2. Antimicrobial Susceptibility Profile
3.3. Detection of Beta-Lactam Resistance, Heavy Metal Resistance Genes, and Virulence Genes
3.4. Physical-Chemical Analysis
3.5. Correlations between Environmental Parameters and Vibrio spp. Abundance
3.6. BOX-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Lin, H.; Wang, X.; Austin, B. Significance of Vibrio species in the marine organic carbon cycle—A review. Sci. China Earth Sci. 2018, 61, 1357–1368. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Trinanes, J.; Martinez-Urtaza, J. The new tools revolutionizing Vibrio science. Environ. Microbiol. 2020, 22, 4096–4100. [Google Scholar] [CrossRef] [PubMed]
- Bowley, J.; Baker-Austin, C.; Porter, A.; Hartnell, R.; Lewis, C. Oceanic hitchhikers—Assessing pathogen risks from marine microplastic. Trends Microbiol. 2021, 29, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Genome Taxonomy Database (GTDB). Available online: https://gtdb.ecogenomic.org/tree (accessed on 7 June 2020).
- Baker-Austin, C.; Trinanes, J.; Gonzalez-Escalona, N.; Martinez-Urtaza, J. Non-Cholera Vibrios: The microbial barometer of climate change. Trends Microbiol. 2017, 25, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Regev, Y.; Davidovich, N.; Berzak, R.; Lau, S.C.; Scheinin, A.P.; Tchernov, D.; Morick, D. Molecular identification and characterization of Vibrio species and Mycobacterium species in wild and cultured marine fish from the Eastern Mediterranean Sea. Microorganisms 2020, 8, 863. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Batz, M.B.; Morris, J.G., Jr. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J. Food Prot. 2012, 75, 1292–1302. [Google Scholar] [CrossRef]
- Froelich, B.A.; Daines, D.A. In hot water: Effects of climate change on Vibrio-human interactions. Environ. Microbiol. 2020, 22, 4101–4111. [Google Scholar] [CrossRef] [Green Version]
- Cholera and Other Vibrio Illness Surveillance (COVIS). Available online: https://www.cdc.gov/vibrio/surveillance.html (accessed on 3 April 2021).
- Elmahdi, S.; DaSilva, L.V.; Parveen, S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: A review. Food Microbiol. 2016, 57, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, K.Y.; Letchumanan, V.; Law, J.W.F.; Pusparajah, P.; Goh, B.H.; Ab Mutalib, N.S.; He, Y.W.; Lee, L.H. Incidence of antibiotic resistance in Vibrio spp. Rev. Aquac. 2020, 12, 2590–2608. [Google Scholar] [CrossRef]
- Gillings, M.R.; Paulsen, I.T. Microbiology of the Anthropocene. Anthropocene 2014, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Surette, M.D.; Wright, G.D. Lessons from the environmental antibiotic resistome. Annu. Rev. Microbiol. 2017, 71, 309–329. [Google Scholar] [CrossRef] [PubMed]
- Gregoracci, G.B.; Nascimento, J.R.; Cabral, A.S.; Paranhos, R.; Valentin, J.L.; Thompson, C.C.; Thompson, F.L. Structuring of bacterioplankton diversity in a large tropical bay. PLoS ONE 2012, 7, e31408. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.M.; Maldonado, G.C.; Castro, R.O.; de Sá Felizardo, J.; Cardoso, R.P.; dos Anjos, R.M.; de Araújo, F.V. Dispersal of potentially pathogenic bacteria by plastic debris in Guanabara Bay, RJ, Brazil. Mar. Poll. Bull. 2019, 141, 561–568. [Google Scholar] [CrossRef]
- Programa de Pesquisas Ecológicas de Longa Duração (PELD Guanabara). Available online: http://www.peldguanabara.lncc.br/guanabara.php (accessed on 7 August 2020). (In Portuguese).
- Fistarol, G.O.; Coutinho, F.H.; Moreira, A.P.B.; Venas, T.; Cánovas, A.; de Paula, S.E.M.; Coutinho, R.; de Moura, R.L.; Valentin, J.L.; Tenenbaum, D.R.; et al. Environmental and sanitary conditions of Guanabara Bay, Rio de Janeiro. Front. Microbiol. 2015, 6, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neto, J.A.B.; Barreto, C.F.; Vilela, C.G.; da Fonseca, E.M.; Melo, G.V.; Barth, O.M. Environmental change in Guanabara Bay, SE Brazil, based in microfaunal, pollen and geochemical proxies in sedimentary cores. Ocean Coast. Manag. 2017, 143, 4–15. [Google Scholar] [CrossRef]
- Histórico dos Boletins de Balneabilidade das Praias da Ilha do Governador e de Ramos–2019 (INEA). Available online: http://www.inea.rj.gov.br/wp-content/uploads/2019/03/ilha_do_governador_historico_2019.pdf (accessed on 13 April 2020). (In Portuguese)
- Kopprio, G.A.; Neogi, S.B.; Rashid, H.; Alonso, C.; Yamasaki, S.; Koch, B.P.; Gärdes, A.; Lara, R.J. Vibrio and bacterial communities across a pollution gradient in the bay of Bengal: Unraveling their biogeochemical drivers. Front. Microbiol. 2020, 11, 594. [Google Scholar] [CrossRef] [Green Version]
- Painel Saneamento Brasil. Available online: https://www.painelsaneamento.org.br/localidade/index?id=331 (accessed on 3 April 2021). (In Portuguese).
- Leonard, A.F.; Zhang, L.; Balfour, A.J.; Garside, R.; Gaze, W.H. Human recreational exposure to antibiotic resistant bacteria in coastal bathing waters. Environ. Int. 2015, 82, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.S.; Sapkota, A.R.; Jacobs, J.M.; He, X.; Crump, B.C. Recreational swimmers’ exposure to Vibrio vulnificus and Vibrio parahaemolyticus in the Chesapeake Bay, Maryland, USA. Environ. Int. 2015, 74, 99–105. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; p. 173. [Google Scholar]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; Wiley-VCH: Weinhein, Germany, 2009. [Google Scholar]
- Baird, R.B. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association; American Water Works Association; Water Environment Federation: Denver, CO, USA, 2017. [Google Scholar]
- Chiou, J.; Li, R.; Chen, S. CARB-17 family of β-lactamases mediates intrinsic resistance to penicillins in Vibrio parahaemolyticus. Antimicrob. Agents Chemother. 2015, 59, 3593–3595. [Google Scholar] [CrossRef] [Green Version]
- Reiner, K. Catalase Test Protocol. ASM Microbe Library. 2010. Available online: https://asm.org/getattachment/72a871fc-ba92-4128-a194-6f1bab5c3ab7/Catalase-Test-Protocol.pdf (accessed on 2 February 2021).
- Rodrigues, N.; Bronzato, G.; Santiago, G.; Botelho, L.; Moreira, B.; Coelho, I.; Souza, M.; Coelho, S. The matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) identification versus biochemical tests: A study with enterobacteria from a dairy cattle environment. Braz. J. Microbiol. 2017, 48, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Approved Guideline-Second Edition; CLSI document M45-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010; ISBN 1-56238-732-4. ISSN 0273-3099. Available online: https://clsi.org (accessed on 2 February 2021).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Yao, L.; Li, F.; Tan, Z.; Zhai, Y.; Wang, L. Characterization of antimicrobial resistance of Vibrio parahaemolyticus from cultured sea cucumbers (A. postichopus japonicas). Lett. Appl. Microbiol. 2014, 59, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Jarlier, V.; Nicolas, M.H.; Fournier, G.; Philippon, A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: Hospital prevalence and susceptibility patterns. Clin. Infect. Dis. 1988, 10, 867–878. [Google Scholar] [CrossRef]
- Walsh, P.S.; Metzger, D.A.; Higuchi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotech. 1991, 10, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Neto, J.A.B.; Gingele, F.X.; Leipe, T.; Brehme, I. Spatial distribution of heavy metals in surficial sediments from Guanabara Bay: Rio de Janeiro, Brazil. Environ. Earth Sci. 2006, 49, 1051. [Google Scholar] [CrossRef]
- Covelli, S.; Protopsalti, I.; Acquavita, A.; Sperle, M.; Bonardi, M.; Emili, A. Spatial variation, speciation and sedimentary records of mercury in the Guanabara Bay (Rio de Janeiro, Brazil). Cont. Shelf Res. 2012, 35, 29–42. [Google Scholar] [CrossRef]
- Versalovic, J.; Schneider, M.; De Bruijn, F.J.; Lupski, J.R. Genomic fingerprinting of bacteria using repetitive sequence based polymerase chain reaction. Methods Mol. Cell. Biol. 1994, 5, 25–40. [Google Scholar]
- Li, Y.; Xie, T.; Pang, R.; Wu, Q.; Zhang, J.; Lei, T.; Xue, L.; Wu, H.; Wang, J.; Ding, Y.; et al. Food-Borne Vibrio parahaemolyticus in China: Prevalence, Antibiotic Susceptibility, and Genetic Characterization. Front. Microbiol. 2020, 11, 1670. [Google Scholar] [CrossRef]
- Raghunath, P. Roles of thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) in Vibrio parahaemolyticus. Front. Microbiol. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Lüdeke, C.H.; Bowers, J.C.; Garret, N.; Fischer, M.; Parsons, M.B.; Bopp, C.A.; DePaola, A. Biochemical, serological, and virulence characterization of clinical and oyster Vibrio parahaemolyticus isolates. J. Clin. Microbiol. 2012, 50, 2343–2352. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Ilyas, S.; Hall, J.A.; Jones, S.H.; Cooper, V.S.; Whistler, C.A. Genetic characterization of clinical and environmental Vibrio parahaemolyticus from the Northeast USA reveals emerging resident and non-indigenous pathogen lineages. Front. Microbiol. 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.C.; Gerding, M.J.; Jones, S.H.; Whistler, C.A. Comparison of the pathogenic potentials of environmental and clinical Vibrio parahaemolyticus strains indicates a role for temperature regulation in virulence. Appl. Environ. Microbiol. 2010, 76, 7459–7465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, L.O.; de Lanna, C.A.; Arcanjo, A.; Bisch, P.M.; von Krüger, W. Genotypic diversity and pathogenic potential of clinical and environmental Vibrio parahaemolyticus isolates from Brazil. Front. Microbiol. 2021, 12, 602653. [Google Scholar] [CrossRef]
- Montánchez, I.; Ogayar, E.; Plágaro, A.H.; Esteve-Codina, A.; Gómez-Garrido, J.; Orruño, M.; Arana, I.; Kaberdin, V.R. Analysis of Vibrio harveyi adaptation in sea water microcosms at elevated temperature provides insights into the putative mechanisms of its persistence and spread in the time of global warming. Sci. Rep. 2019, 9, 289. [Google Scholar] [CrossRef] [Green Version]
- Ford, C.L.; Powell, A.; Lau, D.; Turner, A.D.; Dhanji-Rapkova, M.; Martinez-Urtaza, J.; Baker-Austin, C. Isolation and characterization of potentially pathogenic Vibrio species in a temperate, higher latitude hotspot. Environ. Microbiol. Rep. 2020, 12, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Montánchez, I.; Kaberdin, V.R. Vibrio harveyi: A brief survey of general characteristics and recent epidemiological traits associated with climate change. Mar. Environ. Res. 2020, 154, 104850. [Google Scholar] [CrossRef]
- Motlagh, H.R.A.; Farhangi, M.; Rafiee, G.; Noori, F. Modulating gut microbiota and digestive enzyme activities of Artemia urmiana by administration of different levels of Bacillus subtilis and Bacillus licheniformis. Aquac. Int. 2012, 20, 693–705. [Google Scholar] [CrossRef]
- Da Silva, B.C.; Vieira, F.D.N.; Mouriño, J.L.P.; Ferreira, G.S.; Seiffert, W.Q. Salts of organic acids selection by multiple characteristics for marine shrimp nutrition. Aquaculture 2013, 384, 104–110. [Google Scholar] [CrossRef]
- Sahu, K.K.; Sherif, A.A.; Davaro, R. A rare cause of cellulitis: Photobacterium damselae. J. Microsc. Ultrastruct. 2020, 8, 25. [Google Scholar] [CrossRef]
- Batra, P.; Mathur, P.; Misra, M.C. Aeromonas spp.: An emerging nosocomial pathogen. J. Lab. Physicians 2016, 8, 001–004. [Google Scholar] [CrossRef]
- Lafisca, A.; Pereira, C.S.; Giaccone, V.; Rodrigues, D.D.P. Enzymatic characterization of Vibrio alginolyticus strains isolated from bivalves harvested at Venice Lagoon (Italy) and Guanabara Bay (Brazil). Rev. Inst. Med. Trop. São Paulo. 2008, 50, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Kriem, M.R.; Banni, B.; El Bouchtaoui, H.; Hamama, A.; El Marrakchi, A.; Chaouqy, N.; Pillot, R.; Quilici, M.L. Prevalence of Vibrio spp. in raw shrimps (Parapenaeus longirostris) and performance of a chromogenic medium for the isolation of Vibrio strains. Lett. Appl. Microbiol. 2015, 61, 224–230. [Google Scholar] [CrossRef]
- Antibiotic Resistance–World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 15 June 2020).
- Zago, V.; Veschetti, L.; Patuzzo, C.; Malerba, G.; Lleo, M.M. Resistome mobilome and virulome analysis of Shewanella algae and Vibrio spp. strains isolated in Italian aquaculture centers. Microorganisms 2020, 8, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordmann, P.; Poirel, L.; Walsh, T.R.; Livermore, D.M. The emerging NDM carbapenemases. Trends Microbiol. 2011, 19, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Bier, N.; Schwartz, K.; Guerra, B.; Strauch, E. Survey on antimicrobial resistance patterns in Vibrio vulnificus and Vibrio cholerae non-O1/non-O139 in Germany reveals carbapenemase-producing Vibrio cholerae in coastal waters. Front. Microbiol. 2015, 6, 1179. [Google Scholar] [CrossRef] [PubMed]
- Hammerl, J.A.; Jäckel, C.; Bortolaia, V.; Schwartz, K.; Bier, N.; Hendriksen, R.S.; Guerra, B.; Strauch, E. Carbapenemase VCC-1-producing Vibrio cholerae in coastal waters of Germany. Emerg. Infect. Dis. 2017, 23, 1735–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial pharmaceuticals in the aquatic environment-occurrence and environmental implications. Eur. J. Pharmacol. 2020, 866, 172813. [Google Scholar] [CrossRef]
- Montezzi, L.F.; Campana, E.H.; Corrêa, L.L.; Justo, L.H.; Paschoal, R.P.; da Silva, I.L.V.D.; Souza, M.C.M.; Drolshagen, M.; Picão, R.C. Occurrence of carbapenemase-producing bacteria in coastal recreational waters. Int. J. Antimicrob. Agents. 2015, 45, 174–177. [Google Scholar] [CrossRef]
- Chow, L.; Waldron, L.; Gillings, M. Potential impacts of aquatic pollutants: Sub-clinical antibiotic concentrations induce genome changes and promote antibiotic resistance. Front. Microbiol. 2015, 6, 803. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, A.W.; Power, A.; Hansen, M.G.; Brandt, K.K.; Piliposian, G.; Appleby, P.; O’Neill, P.A.; Jones, R.T.; Sierocinski, P.; Koskella, B.; et al. Heavy metal pollution and co-selection for antibiotic resistance: A microbial palaeontology approach. Environ. Int. 2019, 32, 105117. [Google Scholar] [CrossRef]
- Aguiar, V.M.D.C.; de Lima, M.N.; Abuchacra, R.C.; Abuchacra, P.F.; Neto, J.A.; Borges, H.V.; de Oliveira, V.C. Ecological risks of trace metals in Guanabara Bay, Rio de Janeiro, Brazil: An index analysis approach. Ecotoxicol. Environ. Saf. 2016, 133, 306–315. [Google Scholar] [CrossRef]
- Dahanayake, P.S.; Hossain, S.; Wickramanayake, M.V.K.S.; Heo, G.J. Prevalence of virulence and Extended-Spectrum β-Lactamase (ESBL) genes harboring Vibrio spp. isolated from cockles (Tegillarca granosa) marketed in Korea. Lett. Appl. Microbiol. 2019, 71, 61–69. [Google Scholar] [CrossRef]
- Hossain, S.; Wickramanayake, M.V.K.S.; Dahanayake, P.S.; Heo, G.J. Occurrence of virulence and extended-spectrum β-lactamase determinants in Vibrio spp. isolated from marketed hard-shelled mussel (Mytilus coruscus). Microb. Drug Resist. 2020, 26, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Fresia, P.; Antelo, V.; Salazar, C.; Giménez, M.; D’Alessandro, B.; Afshinnekoo, E.; Mason, C.; Gonnet, G.H.; Iraola, G. Urban metagenomics uncover antibiotic resistance reservoirs in coastal beach and sewage waters. Microbiome 2019, 7, 35. [Google Scholar] [CrossRef]
- Hackbusch, S.; Wichels, A.; Gimenez, L.; Döpke, H.; Gerdts, G. Potentially human pathogenic Vibrio spp. in a coastal transect: Occurrence and multiple virulence factors. Sci. Total Environ. 2020, 707, 136113. [Google Scholar] [CrossRef] [PubMed]
- Gyraite, G.; Kataržytė, M.; Overlingė, D.; Vaičiūtė, D.; Jonikaitė, E.; Schernewski, G. Skip the Dip—Avoid the risk? Integrated microbiological water quality assessment in the South-Eastern Baltic Sea coastal waters. Water 2020, 12, 3146. [Google Scholar] [CrossRef]
- Siboni, N.; Balaraju, V.; Carney, R.; Labbate, M.; Seymour, J.R. Spatiotemporal dynamics of Vibrio spp. within the Sydney Harbour estuary. Front. Microbiol. 2016, 7, 460. [Google Scholar] [CrossRef]
- Coutinho, F.H.; Thompson, C.C.; Cabral, A.S.; Paranhos, R.; Dutilh, B.E.; Thompson, F.L. Modelling the influence of environmental parameters over marine planktonic microbial communities using artificial neural networks. Sci. Total Environ. 2019, 677, 205–214. [Google Scholar] [CrossRef]
- WHO Recommendations on Scientific, Analytical and Epidemiological Developments Relevant to the Parameters for Bathing Water Quality in the Bathing Water Directive (2006/7/EC). Available online: https://circabc.europa.eu/d/d/workspace/SpacesStore/9e89152c-7cfe-4391-9bcf-c173519e8181/WHO%20Recommendations%20on%20EC%20BWD.pdf (accessed on 5 April 2021).
- Asplund, M.E.; Rehnstam-Holm, A.S.; Atnur, V.; Raghunath, P.; Saravanan, V.; Härnström, K.; Collin, B.; Karunasagar, I.; Godhe, A. Water column dynamics of Vibrio in relation to phytoplankton community composition and environmental conditions in a tropical coastal area. Environ. Microbiol. 2011, 13, 2738–2751. [Google Scholar] [CrossRef] [Green Version]
- CONAMA—Conselho Nacional do Meio Ambiente, Resolução no 274 de 29 de NOVEMBRO de 2000. Estabelece Condições de Balneabilidade das ÁGUAS BRASILEIRAS. Brasília. Available online: http://www.mma.gov.br/port/conama/legiabre.cfm?codlegi=272 (accessed on 17 June 2020). (In Portuguese)
- Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of Bathing water Quality and Repealing Directive 76/160/EEC. Available online: https://eur-lex.europa.eu/eli/dir/2006/7/oj (accessed on 5 April 2021).
- Guidelines for Safe Recreational Water Environments. Volume 1, Coastal and Fresh Waters. World Health Organization. 2003. Available online: https://apps.who.int/iris/bitstream/handle/10665/42591/9241545801.pdf?sequence=1&isAllowed=y (accessed on 5 April 2021).
- Olivatto, G.P.; Martins, M.C.T.; Montagner, C.C.; Henry, T.B.; Carreira, R.S. Microplastic contamination in surface waters in Guanabara Bay, Rio de Janeiro, Brazil. Mar. Poll. Bull. 2019, 139, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kesy, K.; Labrenz, M.; Scales, B.S.; Kreikemeyer, B.; Oberbeckmann, S. Vibrio colonization is highly dynamic in early microplastic-associated biofilms as well as on field-collected microplastics. Microorganisms 2021, 9, 76. [Google Scholar] [CrossRef] [PubMed]




| Antimicrobial | Number of Isolates | ||
|---|---|---|---|
| S 1 | I 2 | R 3 | |
| Amikacin | 116 (78.0%) | 12 (8.0%) | 21 (14.0%) |
| Cefotaxime | 111 (74.5%) | 11 (7.4%) | 27 (18.1%) |
| Imipenem | 147 (98.6%) | 0 | 2 (1.4%) |
| Amoxicillin-clavulanic acid | 133 (89.3%) | 7 (4.7%) | 9 (6.0%) |
| Ciprofloxacin | 135 (90.6%) | 11 (7.4%) | 3 (2.0%) |
| Ceftazidime | 134 (89.9%) | 4 (2.7%) | 11 (7.4%) |
| Tetracycline | 147 (98.6%) | 1 (0.7%) | 1 (0.7%) |
| Trimethoprim-sulfamethoxazole | 146 (98.0%) | 0 | 3 (2.0%) |
| Strains | Antimicrobial Non-Susceptibility | Resistance Genes | MAR Index |
|---|---|---|---|
| V. harveyi B7F-82 * | AMI, CAZ, CIP, CTX | blaTEM, blaSHV, merA, A1F/A1R | 0.500 |
| V. parahaemolyticus B34S-4 1 | CAZ, CTX | blaGES, blaCTX-M-1,2, copA, cusB | 0.250 |
| V. parahaemolyticus B34F-4 1 * | AMC, AMI, CAZ, CIP, CTX, IPM, TET | blaSHV, merA, A1F/A1R | 0.875 |
| V. harveyi B34F-73 | AMC, AMI, CTX | blaTEM, blaCTX-M-1,2, copA | 0.375 |
| Environmental Parameters | Sampling Sites in the GB * | ||
|---|---|---|---|
| Site 1 | Site 7 | Site 34 | |
| Water temperature (°C) | 16.80–27.22 (22.80 ± 3.09) | 17.30–28.60 (23.61 ± 3.30) | 22.60–31.00 (26.47 ± 2.59) |
| Air Temperature (°C) | 21.70–31.80 (26.70 ± 2.70) | 21.00–30.0 (26.45 ± 2.83) | 24.60–33.50 (28.95 ± 2.71) |
| Water transparency (m) | 1.40–5.80 (2.94 ± 1.33) | 0.60–5.80 (1.79 ± 1.23) | 0.40–1.05 (0.73 ± 0.19) |
| Salinity (S) | 30.08–35.73 (33.8 ± 1.39) | 26.28–35.10 (32.7 ± 2.21) | 12.61–31.68 (26.12 ± 5.30) |
| Total phosphorus (µmol/L) | 0.75–2.74 (1.43 ± 0.57) | 0.89–7.92 (2.34 ± 1.82) | 4.59–28.17 (13.01 ± 7.07) |
| Total nitrogen (µmol/L) | 8.84–44.53 (24.61 ± 9.69) | 9.73–150.35 (44.32 ± 37.7) | 90.63–588.66 (280.85 ± 156.39) |
| Chlorophyll (µg/L) | 1.07–28.07 (8.70 ± 7.67) | 0.94–193.13 (22.60 ± 43.27) | 7.02–310.07 (79.52 ± 87.20) |
| Pheophytin (µg/L) | 1.46–7.17 (3.17 ± 1.59) | 2.28–19.25 (5.46 ± 4.02) | 0.94–48.18 (15.81 ± 12.33) |
| Thermotolerant coliforms (MPN/100 mL) | 2–700 (109.95 ± 172.38) | 2– 1600 (137.03 ± 365.89) | 18–920,000 (70,432.18 ± 198,491.08) |
| Total coliforms (MPN/100 mL) | 2–9200 (539.31 ± 1941.74) | 2–1600 (163.42 ± 371.05) | 18–920,000 (89,111.27 ± 207,852.28) |
| E. coli (MPN/100 mL) | 13–330 (68.36 ± 87.98) | 2–920 (99.76 ± 246.47) | 18–920,000 (58,911.72 ± 196,373.66) |
| Heterotrophic bacteria (CFU/mL) | 10–244,500 (15,532.63 ± 54,988.25) | 18–82,500 (4142.5 ± 17,526.08) | 465–1,360,000 (143,427.5 ± 331,527.87) |
| Environmental Parameters | Antimicrobial Non-Susceptible Vibrio spp. | |||||||
|---|---|---|---|---|---|---|---|---|
| August 2018 | January 2019 | February 2019 | May 2019 | |||||
| r | p | r | p | r | p | r | p | |
| Air temperature | 0.837 * | 0.038 | −0.542 | 0.266 | −0.612 | 0.196 | 0.820 * | 0.046 |
| Water temperature | 0.789 | 0.062 | −0.840 * | 0.036 | −0.693 | 0.127 | 0.956 ** | 0.003 |
| Salinity | 0.867 * | 0.025 | 0.663 | 0.151 | 0.880 * | 0.021 | −0.878 * | 0.021 |
| Total phosphorus | 0.865 * | 0.026 | −0.517 | 0.293 | −0.830 * | 0.041 | 0.961 ** | 0.002 |
| Total nitrogen | 0.862 * | 0.027 | −0.507 | 0.305 | −0.814 * | 0.049 | 0.955 ** | 0.003 |
| Water transparency | −0.459 | 0.359 | −0.353 | 0.493 | 0.698 | 0.123 | −0.558 | 0.250 |
| Chlorophyll | 0.866 * | 0.026 | −0.512 | 0.299 | −0.852 * | 0.031 | 0.817 * | 0.047 |
| Pheophytin | 0.263 | 0.615 | −0.583 | 0.225 | −0.791 | 0.061 | 0.152 | 0.774 |
| Thermotolerant coliforms | 0.804 | 0.054 | −0.533 | 0.276 | −0.775 | 0.070 | 0.936 ** | 0.006 |
| Total coliforms | 0.804 | 0.054 | −0.533 | 0.276 | −0.775 | 0.070 | 0.948 ** | 0.004 |
| E. coli | 0.807 | 0.052 | −0.533 | 0.276 | −0.775 | 0.070 | 0.946 ** | 0.004 |
| Heterotrophic bacteria | 0.801 | 0.055 | −0.449 | 0.371 | −0.790 | 0.061 | 0.944 ** | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canellas, A.L.B.; Lopes, I.R.; Mello, M.P.; Paranhos, R.; de Oliveira, B.F.R.; Laport, M.S. Vibrio Species in an Urban Tropical Estuary: Antimicrobial Susceptibility, Interaction with Environmental Parameters, and Possible Public Health Outcomes. Microorganisms 2021, 9, 1007. https://doi.org/10.3390/microorganisms9051007
Canellas ALB, Lopes IR, Mello MP, Paranhos R, de Oliveira BFR, Laport MS. Vibrio Species in an Urban Tropical Estuary: Antimicrobial Susceptibility, Interaction with Environmental Parameters, and Possible Public Health Outcomes. Microorganisms. 2021; 9(5):1007. https://doi.org/10.3390/microorganisms9051007
Chicago/Turabian StyleCanellas, Anna L. B., Isabelle R. Lopes, Marianne P. Mello, Rodolfo Paranhos, Bruno F. R. de Oliveira, and Marinella S. Laport. 2021. "Vibrio Species in an Urban Tropical Estuary: Antimicrobial Susceptibility, Interaction with Environmental Parameters, and Possible Public Health Outcomes" Microorganisms 9, no. 5: 1007. https://doi.org/10.3390/microorganisms9051007