Parasitological Survey in European Brown Hare (Lepus europaeus Pallas, 1778) Breeding Facilities in Southern Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Coprological Sampling
2.2. Coprological Investigations
2.3. Statistical Analysis
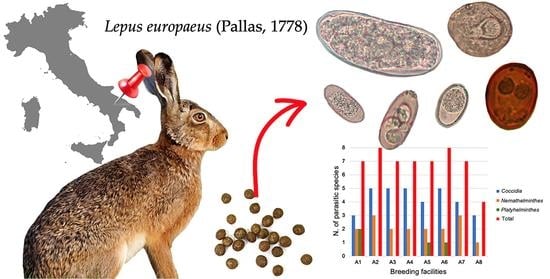
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mori, E.; Carbone, R.; Viviano, A.; Calosi, M.; Fattorini, N. Factors affecting spatiotemporal behaviour in the European brown hare Lepus europaeus: A meta-analysis. Mamm. Rev. 2022, 52, 454–470. [Google Scholar] [CrossRef]
- Riga, F.; Trocchi, V.; Randi, E.; Toso, S. Morphometric differentiation between the Italian hare (Lepus corsicanus De Winton, 1898) and the European brown hare (Lepus europaeus Pallas, 1778). J. Zool. Lond. 2001, 253, 241–252. [Google Scholar] [CrossRef]
- Pikula, J.; Beklová, M.; Holešovská, Z.; Treml, F. Ecology of European Brown Hare and Distribution of Natural Foci of Tularaemia in the Czech Republic. Acta Vet. Brno 2004, 73, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Dubinsky, P.; Vasilková, Z.; Hurníková, Z.; Miterpáková, M.; Slamečka, J.; Jurčík, R. Parasitic infections of the European brown hare (Lepus europaeus Pallas, 1778) in south-western Slovakia. Helminthologia 2010, 47, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Lukešová, D.; Langrová, I.; Vadlejch, J.; Jankovskà, I.; Hlava, J.; Vàlek, P.; Čadková, Z. Endoparasites in European hares (Lepus europaeus) under gamekeeping conditions in the Czech Republic. Helminthologia 2012, 49, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Chorust, K.; Vodnansky, M.; Pikula, J. Parasite load of European brown hares in Austria and the Czech Republic. Vet. Med. 2012, 57, 551–558. [Google Scholar] [CrossRef] [Green Version]
- Diakou, A.; Sokos, C.; Papadopoulos, E. Endoparasites found in European brown hares (Lepus europaeus) hunted in Macedonia, Greece. Helminthologia 2014, 51, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Sergi, V.; Romeo, G.; Serafini, M.; Torretta, E.; Macchioni, F. Endoparasites of the European hare (Lepus europaeus) (Pallas, 1778) in central Italy. Helminthologia 2018, 55, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Rusu, S. Parasitic fauna at the hare (Lepus europaeus Pallas, 1778) from the “Codrii” natural reservation, Republic of Moldova. Lucr. Stiint. Ser. Med. Vet. 2020, 63, 108–114. [Google Scholar]
- Panayotova-Pencheva, M.S. Endoparasites of the European brown hare (Lepus europaeus Pallas, 1778 L.) (Lagomorpha: Leporidae) from Bulgaria. Ann. Parasitol. 2022, 68, 553–562. [Google Scholar]
- Bizzarri, E. Endoparassitosi Delle Lepri Libere e Allevate del Parco Dell’Orecchiella e Variabilità Quali-Quantitativa Della Coccidiosi. Master’s Thesis, Pisa University, Pisa, Italy, 27 October 2006. (In Italian). [Google Scholar]
- Tizzani, P.; Catalano, S.; Rossi, L.; Duignan, P.J.; Meneguz, P.G. Invasive species and their parasites: Eastern cottontail rabbit Sylvilagus floridanus and Trichostrongylus affinis (Graybill, 1924) from Northwestern Italy. Parasitol. Res. 2014, 113, 1301–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacchi, L.; Prigioni, C. Prevalenza e carica parassitaria da coccidi e nematodi in lepri campionate in aree protette della provincia di Pavia. In Proceedings of the Atti II Convegno Nazionale Biologia della Selvaggina, Bologna, Italy, 7 March 1991. (In Italian). [Google Scholar]
- Tacconi, G.; Piergili Fioretti, D.; Moretti, A.; Nobilini, N.; Pasquali, P. Coccidia in Hare Lepus europaeus reared in Umbria, Italy: Bioepidemiological Study. J. Protozool. Res. 1995, 5, 11–85. [Google Scholar]
- Paoletti, B.; Traversa, D.; Levarato, V.; Gatti, A. Identificazione morfologica di Eimeria spp. In lepri (Lepus europaeus) allevate in provincia di Teramo. SUMMA 2010, 1, 31–36. [Google Scholar]
- Dryden, M.W.; Payne, P.A.; Smith, V. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet. Ther. 2005, 6, 15–18. [Google Scholar] [PubMed]
- Ministry of Agriculture Fisheries and Food. Manual of Veterinary Parasitological Techniques, 3rd ed.; Her Majesty’s Stationary Office: London, UK, 1986; p. 160.
- Pellérdy, L.P. Coccidia and Coccidiosis, 1st ed.; Akadémiai Kiadó: Budapest, Hungary, 1965; pp. 358–370. [Google Scholar]
- Levine, N.D.; Ivens, V. Coccidia of the Leporidae. J. Protozool. 1972, 19, 572–581. [Google Scholar] [CrossRef]
- Duranti, E.; Casoli, C. Valorizzazione del territorio con attività diverse da quelle tradizionali. In Proceedings of the Atti Convegno Nazionale “Parliamo di… Allevamenti Alternativi e Valorizzazione del Territorio”, Cuneo, Italy, 25 September 2003. (In Italian). [Google Scholar]
- Alzaga, V.; Tizzani, P.; Acevedo, P.; Ruiz-Fons, F.; Vicente, J.; Gortázar, C. Deviance partitioning of host factors affecting parasitization in the European brown hare (Lepus europaeus). Naturewissenschaften 2009, 96, 1157–1168. [Google Scholar] [CrossRef]
- Soveri, T.; Valtonen, M. Endoparasites of hares (Lepus timidus L. and L. europaeus Pallas) in Finland. J. Wildl. Dis. 1983, 19, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Tenhu, H. Endoparasites of free-living mountain hares (L. timidus varronis) in Switzerland. In Proceedings of the EAZWV Second Scientific Meeting, Chester, UK, 21–24 May 1998. [Google Scholar]
- Aoutil, N.; Bertani, S.; Bordes, F.; Snounou, G.; Chabaud, A.; Landau, I. Eimeria (Coccidia: Eimeridea) of hares in France: Description of a new taxa. Parasite 2005, 12, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Marcato, P.S.; Rosmini, R. Pathology of the Rabbit and Hare: A Color Atlas and Compendium, 1st ed.; Esculapio: Bologna, Italy, 2008. [Google Scholar]
- Kutzer, E.; Frey, H. Die Parasiten der Feldhasen (Lepus europaeus) in Östereich. Berl. Münch. Tierärztl. Wochenschr. 1976, 89, 480–483. (In German) [Google Scholar]
- Sterba, F. Main mortal causes in the European brown hare in 1975-1979. Folia Vet. 1982, 12, 239–260. (In Czech) [Google Scholar]
- McCulloch, C.R.; Prosl, H.; Schmidt, P. A spontaneous and fatal jejunal intussusception in a European brown hare associated with Eimeria leporis. J. Vet. Med. Ser. B 2004, 51, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Chorust, K. Dynamics of coccidial infection in free living and cage-reared European hares. Acta Vet. Brno 1984, 53, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Poglayen, G.; Gaglio, G.; Brianti, D.; Capelli, G. Monitoraggio sanitario della lepre (Lepus europaeus): La fauna parassitaria. In Proceedings of the LXVI Convegno Nazionale SISVet, Rome, Italy, 12 September 2012. (In Italian). [Google Scholar]
- Stancampiano, L.; Geminiani, C.; Trocchi, V. Gastrointestinal helminth community of Lepus europaeus in Bologna province (Emilia-Romagna region): Biodiversity drop in declining populations? In Proceedings of the X Italian Congress of Theriology, Acquapendente, Italy, 20 April 2016. [Google Scholar]
- Newey, S.; Shaw, D.J.; Kirby, A.; Montieth, P.; Hudson, P.J.; Thirgood, S.J. Prevalence, intensity and aggregation of intestinal parasites in mountain hares and their potential impact on population dynamics. Int. J. Parasitol. 2005, 35, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Nigro, M.; Gallazzi, D.; Sironi, G.; Lavazza, A.; Gelmetti, D. Acute hepatosis in European brown hare (Lepus europaeus, Pallas). J. Wildl. Dis. 1991, 27, 621–629. [Google Scholar] [CrossRef] [PubMed]


| Parasite | N. Positive Pools/N. Analyzed Pools (%, 95% C.I.) | Overall Prevalence (%, 95% C.I.) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | ||
| * Cittotenia spp. | 6/27 (22.2%, 8.6–42.3) | 0/21 (0%, 0–16.1) | 0/32 (0%, 0–10.9) | 0/39 (0%, 0–9) | 3/30 (10%, 2.1–26.5) | 3/21 (14.3%, 3–36.3) | 0/40 (0%, 0–8.8) | 0/5 (0%, 0–52.2) | 12/215 (5.6%, 2.9–9.5) |
| * Coccidia | 24/27 (87.5%, 70.8–97.6) | 21/21 (100%, 83.9–100) | 26/32 (81.3%, 63.6–92.8) | 39/39 (100%, 91–100) | 30/30 (100%, 88.4–100) | 19/21 (90.5%, 69.6–98.8) | 33/40 (82.5%, 67.2–92.7) | 5/5 (100%, 47.8–100) | 197/215 (91.2%, 87.1–95) |
|
* Dicrocoelium dendriticum | 3/27 (11.1%, 2.4–29.2) | 0/21 (0%, 0–16.1) | 0/32 (0%, 0–10.9) | 0/39 (0%, 0–9) | 0/30 (0%, 0–11.6) | 0/21 (0%, 0–16.1) | 0/40 (0%, 0–8.8) | 0/5 (0%, 0–52.2) | 3/215 (1.4%, 0.2–5) |
|
** Passalurus ambiguus | 0/27 (0%, 0–12.8) | 5/21 (23.8%, 8.2–47.2) | 0/32 (0%, 0–10.9) | 2/39 (5.1%, 0.6–17.3) | 3/30 (10%, 2.1–26.5) | 5/21 (23.8%, 8.2–47.2) | 5/40 (12.5%, 4.2–26.8) | 0/5 (0%, 0–52.2) | 20/215 (9.3%, 5.8–14) |
|
* Strongyloides papillosus | 5/27 (18.5%, 6.3–38.1) | 3/21 (14.3%, 3–36.3) | 4/32 (12.5%, 3.5–29) | 0/39 (0 %, 0–9) | 0/30 (0%, 0–11.6) | 0/21 (0%, 0–16.1) | 2/40 (5%, 0.6–16.9) | 0/5 (0%, 0–52.2) | 14/215 (6.5%, 3.6–10.7) |
| ** Trichostrongylus retortaeformis | 7/27 (26%, 11.1–46.3) | 7/21 (33.3%, 14.6–57) | 10/32 (31.3%, 16.1–50) | 4/39 (10.3%, 2.9–24.2) | 5/30 (16.6%, 5.6–34.7) | 6/21 (28.6%, 11.3–52.2) | 5/40 (12.5%, 4.2–26.8) | 2/5 (40%, 5.3–85.3) | 46/215 (21.4%, 16.1–27.5) |
| Coccidian Species | N. Positive Pools/N. Analyzed Pools (%, 95% C.I.) | Overall Prevalence (%, 95% C.I.) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | ||
| E. europaea | 0/27 (0%, 0–12.8) | 5/21 (23.8%, 8.2–47.2) | 0/32 (0%, 0–10.9) | 13/39 (33.3%, 19.1–50.2) | 5/30 (16.6%, 5.6–34.7) | 0/21 (0%, 0–16.1) | 0/40 (0%, 0–8.8) | 0/5 (0%, 0–52.2) | 23/215 (10.7%, 6.9–15.6) |
| E. hungarica | 0/27 (0%, 0–12.8) | 3/21 (14.3%, 3–36.3) | 5/32 (15.6%, 5.3–32.8) | 0/39 (0%, 0–9) | 0/30 (0%, 0–11.6) | 6/21 (28.6%, 11.3–52.2) | 0/40 (0%, 0–8.8) | 0/5 (0%, 0–52.2) | 14/215 (6.5%, 3.6–10.7) |
| E. l. leporis | 20/27 (74%, 53.7–88.9) | 20/21 (95.2%, 76.2–99.9) | 25/32 (78.1%, 60–90.7) | 30/39 (77%, 60.7–88.9) | 0/30 (0%, 0–11.6) | 19/21 (90.5%, 69.6–98.8) | 30/40 (75%, 58.8–87.3) | 5/5 (100%, 47.8–100) | 149/215 (69.3%, 62.7–75.4) |
| E. robertsoni | 20/27 (74%, 53.7–88.9) | 21/21 (100%, 83.9–100) | 17/32 (53.1%, 34.7–70.9) | 30/39 (77%, 60.7–88.9) | 16/30 (53.3%, 34.3–71.7) | 8/21 (38.1%, 18.1–61.6) | 20/40 (50%, 33.8–66.2) | 2/5 (40%, 5.3–85.3) | 134/215 (62.3%, 55.5–68.8) |
| E. semisculpta | 10/27 (37.5%, 19.4–57.6) | 0/21 (0%, 0–16.1) | 5/32 (15.6%, 5.3–32.8) | 4/39 (10%, 2.9–24.2) | 8/30 (26.7%, 12.3–45.9) | 8/21 (38.1%, 18.1–61.6) | 5/40 (12.5%, 4.2–26.8) | 0/5 (0%, 0–52.2) | 4/215 (18.6%, 13.6–24.5) |
| E. townsendi | 17/27 (62.5%, 42.4–80.6) | 17/21 (80.9%, 58.1–94.6) | 17/32 (53.1%, 34.7–70.9) | 24/39 (61.5 %, 44.6–76.6) | 26/30 (86.7%, 69.3–96.2) | 13/21 (61.9%, 38.4–81.9) | 13/40 (32.5%, 18.6–49.1) | 2/5 (40%, 5.3–85.3) | 129/215 (60%, 53.1–66.6) |
| Infection Prevalence, Data Source * | |||||||
|---|---|---|---|---|---|---|---|
| Ita1 a | Ita2 b | Ita3 c | Aus d | Cze e | Gre/Mac f | Spa g | Bul h |
| 91.2%, CB | 34.8%, CB 87.7%, W | 34.8%, CB 87.7%, W | 80.4%, W | 79.6%, W | 64.3%, W | 71.7%, W | 55.3%, W |
| Infection Prevalence, Data Source * | |||||||
|---|---|---|---|---|---|---|---|
| Ita1 a | Ita2 b | Ita3 c | Ita4 d | Aus e | Cze f | Gre/Mac g | Spa h |
| 21.4%, CB | 72%, W | 65% | 3%, CB 87.1%, W | 82.7%, W | 83.2%, W | 50%, W | 56.6%, W |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brustenga, L.; Franciosini, M.P.; Diaferia, M.; Rigamonti, G.; Musa, L.; Russomanno, B.L.; Veronesi, F. Parasitological Survey in European Brown Hare (Lepus europaeus Pallas, 1778) Breeding Facilities in Southern Italy. Pathogens 2023, 12, 208. https://doi.org/10.3390/pathogens12020208
Brustenga L, Franciosini MP, Diaferia M, Rigamonti G, Musa L, Russomanno BL, Veronesi F. Parasitological Survey in European Brown Hare (Lepus europaeus Pallas, 1778) Breeding Facilities in Southern Italy. Pathogens. 2023; 12(2):208. https://doi.org/10.3390/pathogens12020208
Chicago/Turabian StyleBrustenga, Leonardo, Maria Pia Franciosini, Manuela Diaferia, Giulia Rigamonti, Laura Musa, Barbara Lidia Russomanno, and Fabrizia Veronesi. 2023. "Parasitological Survey in European Brown Hare (Lepus europaeus Pallas, 1778) Breeding Facilities in Southern Italy" Pathogens 12, no. 2: 208. https://doi.org/10.3390/pathogens12020208