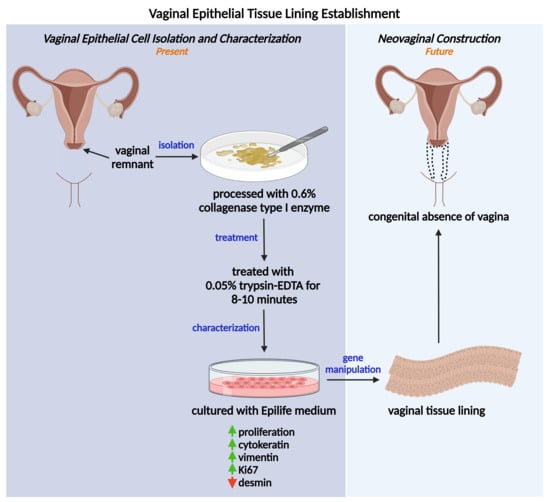
Isolation of Vaginal Epithelial Cells: In Preparation of Autologous Vaginal Tissue Lining for Congenital Absence of Vagina Patients
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Patients with CAV
2.2. Collagenase Demonstrated Effective Tissue Digestion for Primary VEC Isolation
2.3. Primary VECs Revealed Best Epithelial Morphology at Early Passage
2.4. Primary VECs Exhibited High Proliferation Rate
2.5. Primary VECs Exhibited High Vimentin Expression
3. Materials and Methods
3.1. Ethical Approval
3.2. Inpatients with CAV in HCTM, Malaysia
3.3. Vaginal Tissue Processing and Cell Isolation
3.4. Cell Cultures
3.5. Analysis of Cell Proliferation and Viability
3.6. Immunofluorescence Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poonam, P.; Kumar, A.; Sinha, R.R. Mullerian Duct Anomaly- A Spectrum of Varied Clinical Manifestations. Int. J. Contemp. Med. Res. 2019, 6, K10–K13. [Google Scholar] [CrossRef]
- Valappil, S.; Chetan, U.; Wood, N.; Garden, A. Mayer-Rokitansky-Küster-Hauser syndrome: Diagnosis and management. Obstet. Gynaecol. 2012, 14, 93–98. [Google Scholar] [CrossRef]
- Nodale, C.; Vescarelli, E.; D’amici, S.; Maffucci, D.; Ceccarelli, S.; Monti, M.; Panici, P.B.; Romano, F.; Angeloni, A.; Marchese, C. Characterization of Human Vaginal Mucosa Cells for Autologous In Vitro Cultured Vaginal Tissue Transplantation in Patients with MRKH Syndrome. BioMed Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Invitti, A.L.; Takano, C.C.; Rozenchan, P.B.; Vendrame, T.A.P.; Girão, M.J.B.C. Establishment and Characterization of Vaginal Tissue Primary Culture: Feasibility of Cell Therapy for Mayer-Rokitansky-Kuster-Hauser Syndrome (MRKHS) Patient Treatment. J. Biomed. Sci. 2017, 6, 100069. [Google Scholar] [CrossRef]
- Tsunematsu, R.; Minami, C.; Hiasa, K.; Egashira, K.; Kato, K. Successful surgical treatment for congenital vaginal agenesis accompanied by functional uterus: A report of two cases. Gynecol. Minim. Invasive Ther. 2019, 8, 76–79. [Google Scholar] [CrossRef]
- Oliveira, A.F.; Ferreira, H. Neovagina creation in congenital vaginal agenesis: New mini-laparoscopic approach applying intraoperative indocyanine green fluorescence. Surg. Innov. 2019, 28, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-J.; Huang, S.-F.; Lai, J.-Y.; Ma, S.-C.; Chen, H.-C.; Wu, S.-E.; Wang, T.-K.; Sun, C.-C.; Ma, K.S.-K.; Chen, J.-K.; et al. Preservation of epithelial progenitor cells from collagenase-digested oral mucosa during ex vivo cultivation. Sci. Rep. 2016, 6, 36266. [Google Scholar] [CrossRef]
- Autengruber, A.; Gereke, M.; Hansen, G.; Hennig, C.; Bruder, D. Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function. Eur. J. Microbiol. Immunol. 2012, 2, 112–120. [Google Scholar] [CrossRef]
- Skog, M.; Sivlér, P.; Steinvall, I.; Aili, D.; Sjöberg, F.; Elmasry, M. The Effect of Enzymatic Digestion on Cultured Epithelial Autografts. Cell Transplant. 2019, 28, 638–644. [Google Scholar] [CrossRef]
- Huang, H.-L.; Hsing, H.-W.; Lai, T.-C.; Chen, Y.-W.; Lee, T.-R.; Chan, H.-T.; Lyu, P.-C.; Wu, C.-L.; Lu, Y.-C.; Lin, S.-T.; et al. Trypsin-induced proteome alteration during cell subculture in mammalian cells. J. Biomed. Sci. 2010, 17, 36. [Google Scholar] [CrossRef]
- Panchision, D.M.; Chen, H.-L.; Pistollato, F.; Papini, D.; Ni, H.-T.; Hawley, T.S. Optimized Flow Cytometric Analysis of Central Nervous System Tissue Reveals Novel Functional Relationships among Cells Expressing CD133, CD15, and CD24. Stem Cells 2007, 25, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Ng, I.C.; Pawijit, P.; Tan, J.; Yu, H. Anatomy and Physiology for Biomaterials Research and Development. Encycl. Biomed. Eng. 2019, 1, 225–236. [Google Scholar] [CrossRef]
- Petersen, O.W.; Ronnov-Jessen, L.; Howlettt, A.R.; Bissellt, M.J. Interaction with Basement Membrane Serves to Rapidly Distinguish Growth and Differentiation Pattern of Normal and Malignant Human Breast Epithelial Cells (Extracelular Matrix/Rapid Transformation Assay/Breast Cancer/Tissue Structure and Function). Cell Biol. 1992, 89, 9064–9068. [Google Scholar]
- Luciano, M.; Versaevel, M.; Vercruysse, E.; Eonore Vercruysse, E.; Procès, A.; Kalukula, Y.; Remson, A.; Deridoux, A.; Gabriele, S. Appreciating the role of cell shape changes in the mechanobiology of epithelial tissues. Biophys. Rev. 2022, 3, 011305. [Google Scholar] [CrossRef]
- Chennazhy, K.P.; Krishnan, L.K. Effect of passage number and matrix characteristics on differentiation of endothelial cells cultured for tissue engineering. Biomaterials 2005, 26, 5658–5667. [Google Scholar] [CrossRef]
- Simons, J.L.; Vintiner, S.K. Efficacy of Several Candidate Protein Biomarkers in the Differentiation of Vaginal from Buccal Epithelial Cells. J. Forensic. Sci. 2012, 57, 1585–1590. [Google Scholar] [CrossRef]
- Sivagurunathan, S.; Vahabikashi, A.; Yang, H.; Zhang, J.; Vazquez, K.; Rajasundaram, D.; Politanska, Y.; Abdala-Valencia, H.; Notbohm, J.; Guo, M.; et al. Expression of vimentin alters cell mechanics, cell-cell adhesion, and gene expression profiles suggesting the induction of a hybrid EMT in human mammary epithelial cells. Front. Cell Dev. Biol. 2022, 10, 1839. [Google Scholar] [CrossRef]
- Mendez, M.G.; Kojima, S.; Goldman, R.D. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010, 24, 1838–1851. [Google Scholar] [CrossRef]
- Lih Yuan, T.; Sulaiman, N.; Ghani Nur Azurah, A.; Maarof, M.; Adawiyah Razali, R.; Dain Yazid, M.; Dutta, M.; Zhang, L. Oestrogen-induced epithelial-mesenchymal transition (EMT) in endometriosis: Aetiology of vaginal agenesis in Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. Front. Physiol. 2022, 13, 937988. [Google Scholar] [CrossRef]
- Bilyk, O.; Coatham, M.; Jewer, M.; Postovit, L.-M. Epithelial-to-Mesenchymal Transition in the Female Reproductive Tract: From Normal Functioning to Disease Pathology. Front. Oncol. 2017, 7, 145. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Chen, Y.; Chen, S.; Jia, X.; Sun, T.; Liu, Y.; Li, X.; Xiang, R.; Li, N. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of WNT/β-catenin signal network. Cancer Lett. 2013, 336, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.S.; Rota, I.A.; Artibani, M.; Morotti, M.; Hu, Z.; Wietek, N.; Alsaadi, A.; Albukhari, A.; Sauka-Spengler, T.; Ahmed, A.A. Mechanistic Drivers of Müllerian Duct Development and Differentiation Into the Oviduct. Front. Cell Dev. Biol. 2021, 9, 605301. [Google Scholar] [CrossRef]
- Costa, M.L.; Escaleira, R.; Cataldo, A.; Oliveira, F.; Mermelstein, C.S. Desmin: Molecular interactions and putative functions of the muscle intermediate filament protein. Braz. J. Med. Biol. Res. 2004, 37, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Hondo, T.; Kanaya, T.; Tanaka, S.; Takakura, I.; Itani, W.; Rose, M.T.; Kitazawa, H.; Yamaguchi, T.; Aso, H. Characterization of newly established bovine intestinal epithelial cell line. Histochem 2010, 133, 125–134. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lih Yuan, T.; Sulaiman, N.; Nur Azurah, A.G.; Maarof, M.; Razali, R.A.; Koh, B.; Ibrahim, R.; Zainuddin, A.A.; Yazid, M.D. Isolation of Vaginal Epithelial Cells: In Preparation of Autologous Vaginal Tissue Lining for Congenital Absence of Vagina Patients. Int. J. Mol. Sci. 2023, 24, 8798. https://doi.org/10.3390/ijms24108798
Lih Yuan T, Sulaiman N, Nur Azurah AG, Maarof M, Razali RA, Koh B, Ibrahim R, Zainuddin AA, Yazid MD. Isolation of Vaginal Epithelial Cells: In Preparation of Autologous Vaginal Tissue Lining for Congenital Absence of Vagina Patients. International Journal of Molecular Sciences. 2023; 24(10):8798. https://doi.org/10.3390/ijms24108798
Chicago/Turabian StyleLih Yuan, Too, Nadiah Sulaiman, Abdul Ghani Nur Azurah, Manira Maarof, Rabiatul Adawiyah Razali, Benson Koh, Roszita Ibrahim, Ani Amelia Zainuddin, and Muhammad Dain Yazid. 2023. "Isolation of Vaginal Epithelial Cells: In Preparation of Autologous Vaginal Tissue Lining for Congenital Absence of Vagina Patients" International Journal of Molecular Sciences 24, no. 10: 8798. https://doi.org/10.3390/ijms24108798