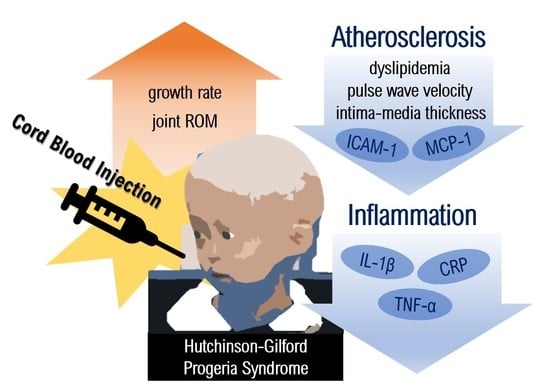
Efficacy of Cord Blood Cell Therapy for Hutchinson–Gilford Progeria Syndrome—A Case Report
Abstract
:1. Introduction
2. Results
2.1. Adverse Events and Deviations from the Protocol
2.2. Anthropometric, Musculoskeletal, and Cognitive Changes after CB Administration
2.3. Amelioration of Atherosclerotic Condition after CB Administration
2.4. Anti-Inflammatory and Anti-Atherosclerotic Responses by CB Cell Therapy
3. Discussion
4. Material and Methods
4.1. General Characteristics
4.2. Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez, S.; Coppede, F.; Sagelius, H.; Eriksson, M. Increased expression of the Hutchinson–Gilford progeria syndrome truncated lamin A transcript during cell aging. Eur. J. Hum. Genet. 2009, 17, 928–937. [Google Scholar] [CrossRef] [Green Version]
- Young, S.G.; Meta, M.; Yang, S.H.; Fong, L.G. Prelamin A farnesylation and progeroid syndromes. J. Biol. Chem. 2006, 281, 39741–39745. [Google Scholar] [CrossRef] [Green Version]
- Cao, K.; Capell, B.C.; Erdos, M.R.; Djabali, K.; Collins, F.S. A lamin A protein isoform overexpressed in Hutchinson–Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc. Natl. Acad. Sci. USA 2007, 104, 4949–4954. [Google Scholar] [CrossRef] [Green Version]
- Hennekam, R.C. Hutchinson-Gilford progeria syndrome: Review of the phenotype. Am. J. Med. Genet. A 2006, 140, 2603–2624. [Google Scholar] [CrossRef] [Green Version]
- Cao, K.; Graziotto, J.J.; Blair, C.D.; Mazzulli, J.R.; Erdos, M.R.; Krainc, D.; Collins, F.S. Rapamycin Reverses Cellular Phenotypes and Enhances Mutant Protein Clearance in Hutchinson-Gilford Progeria Syndrome Cells. Sci. Transl. Med. 2011, 3, 89. [Google Scholar] [CrossRef]
- Bidault, G.; Garcia, M.; Capeau, J.; Morichon, R.; Vigouroux, C.; Béreziat, V. Progerin expression induces inflammation, oxidative stress and senescence in human coronary endothelial cells. Cells 2020, 9, 1201. [Google Scholar] [CrossRef] [PubMed]
- Stehbens, W.E.; Gilbert-Barness, E.; Olson, R.E.; Ackerman, J.J.C.P. Histological and ultrastructural features of atherosclerosis in progeria. Cardiovasc. Pathol. 1999, 8, 29–39. [Google Scholar] [CrossRef]
- Gordon, L.B.; Kleinman, M.E.; Massaro, J.; D’Agostino, R.B.; Shappell, H.; Gerhard-Herman, M.; Smoot, L.B.; Gordon, C.M.; Cleveland, R.H.; Nazarian, A.; et al. Clinical Trial of the Protein Farnesylation Inhibitors Lonafarnib, Pravastatin, and Zoledronic Acid in Children With Hutchinson-Gilford Progeria Syndrome. Circulation 2016, 134, 114–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harhouri, K.; Frankel, D.; Bartoli, C.; Roll, P.; De Sandre-Giovannoli, A.; Lévy, N. An overview of treatment strategies for Hutchinson-Gilford Progeria syndrome. Nucleus 2018, 9, 265–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, L.B.; Shappell, H.; Massaro, J.; D’Agostino, R.B.; Brazier, J.; Campbell, S.E.; Kleinman, M.E.; Kieran, M.W. Association of Lonafarnib Treatment vs No Treatment With Mortality Rate in Patients With Hutchinson-Gilford Progeria Syndrome. JAMA 2018, 319, 1687–1695. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, N.J.; Gordon, L.B. Chapter 18—Hutchinson–Gilford progeria syndrome. In Handbook of Clinical Neurology; Islam, M.P., Roach, E.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 132, pp. 249–264. [Google Scholar]
- Dhillon, S. Lonafarnib: First Approval. Drugs 2021, 81, 283–289. [Google Scholar] [CrossRef]
- Gordon, L.B.; Kleinman, M.E.; Miller, D.T.; Neuberg, D.S.; Giobbie-Hurder, A.; Gerhard-Herman, M.; Smoot, L.B.; Gordon, C.M.; Cleveland, R.; Snyder, B.D. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson–Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 16666–16671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chase, L. The 10 Most Expensive Drugs in the U.S., Period. Available online: https://www.goodrx.com/blog/most-expensive-drugs-period/ (accessed on 6 July 2021).
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Olive, M.; Harten, I.; Mitchell, R.; Beers, J.K.; Djabali, K.; Cao, K.; Erdos, M.R.; Blair, C.; Funke, B.; Smoot, L. Cardiovascular pathology in Hutchinson-Gilford progeria: Correlation with the vascular pathology of aging. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2301–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirwin, T.; Gomes, A.; Amin, R.; Sufi, A.; Goswami, S.; Wang, B. Mechanisms underlying the therapeutic potential of mesenchymal stem cells in atherosclerosis. Regen. Med. 2021, 16, 669–682. [Google Scholar] [CrossRef]
- Cho, K.H.; Choi, J.I.; Kim, J.O.; Jung, J.E.; Kim, D.W.; Kim, M. Therapeutic mechanism of cord blood mononuclear cells via the IL-8-mediated angiogenic pathway in neonatal hypoxic-ischaemic brain injury. Sci. Rep. 2020, 10, 4446. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.; Choi, J.; Kim, M. Combining Human Umbilical Cord Blood Cells With Erythropoietin Enhances Angiogenesis/Neurogenesis and Behavioral Recovery After Stroke. Front. Neurol. 2019, 10, 357. [Google Scholar] [CrossRef]
- Titov, A.; Zmievskaya, E.; Ganeeva, I.; Valiullina, A.; Petukhov, A.; Rakhmatullina, A.; Miftakhova, R.; Fainshtein, M.; Rizvanov, A.; Bulatov, E. Adoptive Immunotherapy beyond CAR T-Cells. Cancers 2021, 13, 743. [Google Scholar] [CrossRef]
- Riordan, N.H.; Chan, K.; Marleau, A.M.; Ichim, T.E. Cord blood in regenerative medicine: Do we need immune suppression? J. Transl. Med. 2007, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Min, K.; Suh, M.R.; Cho, K.H.; Park, W.; Kang, M.S.; Jang, S.J.; Kim, S.H.; Rhie, S.; Choi, J.I.; Kim, H.-J. Potentiation of cord blood cell therapy with erythropoietin for children with CP: A 2 × 2 factorial randomized placebo-controlled trial. Stem Cell Res. Ther. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Min, K.; Song, J.; Kang, J.Y.; Ko, J.; Ryu, J.S.; Kang, M.S.; Jang, S.J.; Kim, S.H.; Oh, D.; Kim, M.K. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: A double-blind, randomized, placebo-controlled trial. Stem Cells 2013, 31, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.; Min, K.; Jang, J.; Kim, S.C.; Kang, M.S.; Jang, S.J.; Lee, J.Y.; Kim, S.H.; Kim, M.K.; An, S.A. Involvement of immune responses in the efficacy of cord blood cell therapy for cerebral palsy. Stem Cells Dev. 2015, 24, 2259–2268. [Google Scholar] [CrossRef]
- Laskowitz, D.T.; Bennett, E.R.; Durham, R.J.; Volpi, J.J.; Wiese, J.R.; Frankel, M.; Shpall, E.; Wilson, J.M.; Troy, J.; Kurtzberg, J. Allogeneic umbilical cord blood infusion for adults with ischemic stroke: Clinical outcomes from a phase I safety study. Stem Cells Transl. Med. 2018, 7, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Reusz, G.S.; Cseprekal, O.; Temmar, M.; Kis, E.; Cherif, A.B.; Thaleb, A.; Fekete, A.; Szabó, A.J.; Benetos, A.; Salvi, P. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension 2010, 56, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kis, É.; Cseprekál, O.; Horváth, Z.; Katona, G.; Fekete, B.C.; Hrapka, E.; Szabó, A.; Szabó, A.J.; Fekete, A.; Reusz, G.S. Pulse wave velocity in end-stage renal disease: Influence of age and body dimensions. Pediatric Res. 2008, 63, 95–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroncini, L.A.V.; Sylvestre, L.d.C.; Pecoits, R. Assessment of intima-media thickness in healthy children aged 1 to 15 years. Arq. Bras. De Cardiol. 2016, 106, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Niboshi, A.; Hamaoka, K.; Sakata, K.; Inoue, F. Characteristics of brachial–ankle pulse wave velocity in Japanese children. Eur. J. Pediatrics 2006, 165, 625–629. [Google Scholar] [CrossRef]
- FOR, E.P.O.I.G.; CHILDREN, R.R.I. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128, S213. [Google Scholar]
- Colantonio, D.A.; Kyriakopoulou, L.; Chan, M.K.; Daly, C.H.; Brinc, D.; Venner, A.A.; Pasic, M.D.; Armbruster, D.; Adeli, K. Closing the gaps in pediatric laboratory reference intervals: A CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin. Chem. 2012, 58, 854–868. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.; Holland, C.; Spencer, R.; Jackson, J.; O’connor, G.; O’donnell, J. Haematological reference ranges for schoolchildren. Clin. Lab. Haematol. 1997, 19, 1–15. [Google Scholar] [CrossRef]
- Oswald, E.; Stalzer, B.; Heitz, E.; Weiss, M.; Schmugge, M.; Strasak, A.; Innerhofer, P.; Haas, T. Thromboelastometry (ROTEM®) in children: Age-related reference ranges and correlations with standard coagulation tests. Br. J. Anaesth. 2010, 105, 827–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desbaillets, I.; Diserens, A.-C.; Tribolet, N.D.; Hamou, M.-F.; Meir, E.G.V. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J. Exp. Med. 1997, 186, 1201–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merideth, M.A.; Gordon, L.B.; Clauss, S.; Sachdev, V.; Smith, A.C.; Perry, M.B.; Brewer, C.C.; Zalewski, C.; Kim, H.J.; Solomon, B. Phenotype and course of Hutchinson–Gilford progeria syndrome. N. Engl. J. Med. 2008, 358, 592–604. [Google Scholar] [CrossRef] [Green Version]
- Turan, S.; Topcu, B.; Gökçe, I.; Güran, T.; Atay, Z.; Omar, A.; Akçay, T.; Bereket, A. Serum alkaline phosphatase levels in healthy children and evaluation of alkaline phosphatasez-scores in different types of rickets. J. Clin. Res. Pediatric Endocrinol. 2011, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Crofton, P.M.; Stirling, H.F.; Kelnar, C. Bone alkaline phosphatase and height velocity in short normal children undergoing growth-promoting treatments: Longitudinal study. Clin. Chem. 1995, 41, 672–678. [Google Scholar] [CrossRef]
- Sato-Kawano, N.; Takemoto, M.; Okabe, E.; Yokote, K.; Matsuo, M.; Kosaki, R.; Ihara, K. The clinical characteristics of Asian patients with classical-type Hutchinson–Gilford progeria syndrome. J. Hum. Genet. 2017, 62, 1031–1035. [Google Scholar] [CrossRef]
- Gordon, L.B.; McCarten, K.M.; Giobbie-Hurder, A.; Machan, J.T.; Campbell, S.E.; Berns, S.D.; Kieran, M.W. Disease progression in Hutchinson-Gilford progeria syndrome: Impact on growth and development. Pediatrics 2007, 120, 824–833. [Google Scholar] [CrossRef]
- Gordon, C.M.; Gordon, L.B.; Snyder, B.D.; Nazarian, A.; Quinn, N.; Huh, S.; Giobbie-Hurder, A.; Neuberg, D.; Cleveland, R.; Kleinman, M. Hutchinson-gilford progeria is a skeletal dysplasia. J. Bone Miner. Res. 2011, 26, 1670–1679. [Google Scholar] [CrossRef] [Green Version]
- Nordmark, E.; Hägglund, G.; Lauge-Pedersen, H.; Wagner, P.; Westbom, L. Development of lower limb range of motion from early childhood to adolescence in cerebral palsy: A population-based study. BMC Med. 2009, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, N.; Gordon, L. Chapter 18-Hutchinson–Gilford progeria syndrome. Neurocutaneous Syndromes. Handb. Clin. Neurol. 2015, 132, 249–264. [Google Scholar]
- Cruickshank, K.; Riste, L.; Anderson, S.G.; Wright, J.S.; Dunn, G.; Gosling, R.G. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: An integrated index of vascular function? Circulation 2002, 106, 2085–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simova, I. Intima-media thickness: Appropriate evaluation and proper measurement. Eur. Soc. Cardiol. Ej Cardiol. Pr. 2015, 13, 21. [Google Scholar]
- Gordon, L.B.; Harten, I.A.; Patti, M.E.; Lichtenstein, A.H. Reduced adiponectin and HDL cholesterol without elevated C-reactive protein: Clues to the biology of premature atherosclerosis in Hutchinson-Gilford Progeria Syndrome. J. Pediatrics 2005, 146, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Talayero, B.G.; Sacks, F.M. The role of triglycerides in atherosclerosis. Curr. Cardiol. Rep. 2011, 13, 544–552. [Google Scholar] [CrossRef] [Green Version]
- Bottazzi, B.; Doni, A.; Garlanda, C.; Mantovani, A. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu. Rev. Immunol. 2009, 28, 157–183. [Google Scholar] [CrossRef]
- Rubinstein, P.; Dobrila, L.; Rosenfield, R.E.; Adamson, J.W.; Migliaccio, G.; Migliaccio, A.R.; Taylor, P.E.; Stevens, C.E. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc. Natl. Acad. Sci. USA 1995, 92, 10119–10122. [Google Scholar] [CrossRef] [Green Version]


| Reference Value | −1 M | 0 M | 4 M | 8 M | 12 M | |
|---|---|---|---|---|---|---|
| Triglyceride (mg/dL) | <90 [30] | 71 | 226 | 172 | 125 | 101 |
| HDL cholesterol (mg/dL) | >45 [30] | 72.7 | 51.8 | 56.7 | 61.4 | 67.3 |
| LDL cholesterol (mg/dL) | <110 [30] | 132 | 101 | 100 | 128 | 102 |
| Total cholesterol (mg/dL) | <170 [30] | 192 | 165 | 183 | 195 | 182 |
| ALP (IU/L) | 127.2–517.2 [31] | 144 | 164 | 178 | 203 | 201 |
| Platelet (103/μL) | 183–370 [32] | 478 | 393 | 421 | 425 | 418 |
| Prothrombin time (sec) | 12.0–13.2 [33] | 13.9 | 13.6 | 13.6 | 12.9 | 13.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, M.R.; Lim, I.; Kim, J.; Yang, P.-S.; Choung, J.S.; Sim, H.R.; Ha, S.C.; Kim, M. Efficacy of Cord Blood Cell Therapy for Hutchinson–Gilford Progeria Syndrome—A Case Report. Int. J. Mol. Sci. 2021, 22, 12316. https://doi.org/10.3390/ijms222212316
Suh MR, Lim I, Kim J, Yang P-S, Choung JS, Sim HR, Ha SC, Kim M. Efficacy of Cord Blood Cell Therapy for Hutchinson–Gilford Progeria Syndrome—A Case Report. International Journal of Molecular Sciences. 2021; 22(22):12316. https://doi.org/10.3390/ijms222212316
Chicago/Turabian StyleSuh, Mi Ri, Ikhyun Lim, Jongwook Kim, Pil-Sung Yang, Jin Seung Choung, Hye Ryeong Sim, Sung Chan Ha, and MinYoung Kim. 2021. "Efficacy of Cord Blood Cell Therapy for Hutchinson–Gilford Progeria Syndrome—A Case Report" International Journal of Molecular Sciences 22, no. 22: 12316. https://doi.org/10.3390/ijms222212316