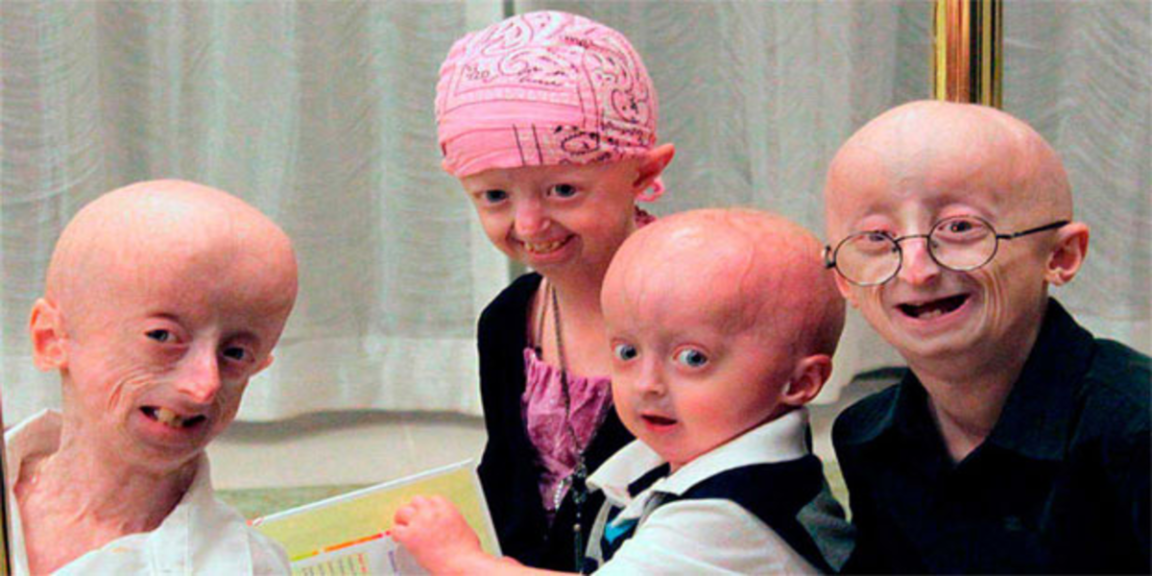
FDA approves the first drug for Hutchinson-Gilford Progeria Syndrome (HGPS)
Hutchinson-Gilford progeria syndrome (#HGPS) is an extremely rare (prevalence 1 in 20 million births), autosomal dominant genetic disorder characterized by premature agin. Children with progeria usually develop the first symptoms during their first few months of life. The earliest symptoms may include a failure to thrive and a localized scleroderma-like skin condition. As a child ages past infancy, additional conditions become apparent, usually around 18–24 months. Limited growth, full-body alopecia (hair loss), and a distinctive appearance (a small face with a shallow, recessed jaw and a pinched nose) are all characteristics of progeria. Signs and symptoms of this progressive disease tend to become more marked as the child ages. Later, the condition causes wrinkled skin, kidney failure, loss of eyesight, and atherosclerosis and other cardiovascular problems. Scleroderma, a hardening and tightening of the skin on trunk and extremities of the body, is prevalent. People diagnosed with this disorder usually have small, fragile bodies, like those of older adults. The head is usually large to the body, with a narrow, wrinkled face and a beak nose. Prominent scalp veins are noticeable (made more obvious by alopecia), as well as prominent eyes. Musculoskeletal degeneration causes loss of body fat and muscle, stiff joints, hip dislocations, and other symptoms generally absent in the non-elderly population. Individuals usually retain typical mental and motor development.
#Lonafarnib is an orally active farnesyltransferase inhibitor being developed by #EigerBioPharmaceuticals under license from Merck & Co. for the treatment of progeria and progeroid laminopathies. Received its frst approval on 20 November 2020 in the USA. The approval of Lonafarnib is for the reduction of the risk of mortality in HGPS and for the treatment of processing-defcient progeroid laminopathies in patients ≥ 12 months. Lonafarnib (SCH 66336) is a nonpeptidic, CAAX-competitive, selective FTase inhibitors (half maximal inhibitory concentration 1.9 nmol/L. In an in vitro study, blocking farnesylation of progerin-transfected cells with lonafarnib restored normal nuclear architecture, and treatment of human HGPS fbroblasts with Lonafarnib resulted in signifcant (p < 0.001) reduction in nuclear blebbing. These results suggest that treatment with lonafarnib represents a potential therapy for patients with HGPS(p< 0,001) reduction in nuclear blebbing. These results suggest that treatment with lonafarnib represents a potential therapy for patients with HGPS.
--
8moI’m reaching out today with an exciting speaking opportunity for your consideration. I am honoured to invite you to speak at [6th International Conference on Rare Diseases], an event focused on World Rare Diseases 2023 in October 04, 2023 as a Webinar