Charuta Gavankar1, Ryan A Grant1*, Robert Fulbright2, Anita Huttner3, Jennifer Moliterno1
1Department of Neurosurgery, Yale University School of Medicine, New Haven, CT, USA
2Department of Radiology, Yale University School of Medicine, New Haven, CT, USA
3Department of Pathology, Yale University School of Medicine, New Haven, CT, USA
*Corresponding Author:
Ryan Grant
Department of Neurosurgery
Yale School of Medicine
333 Cedar Street, TMP4
New Haven, CT 06510, USA
Tel: 203.785.2805
Fax: 203.785.6916
E-mail: ryan.grant@yale.edu
Citation: Gavankar C, Grant RA, Fulbright R, et al. Mixed Tumor with Subependymoma and Ependymoma Features: A Case Report and Review of the Literature. J Neurol Neurosci. 2016, 6:3. doi: 10.21767/2171-6625.100026
Received Date: August 25, 2015; Accepted Date: November 10, 2015; Published Date: November 14, 2015
Keywords
Mixed subependymoma ependymoma; Subependymoma; Ependymoma
Introduction
Subependymomas are rare, slow-growing, benign ependymal neoplasms histologically characterized as grade I tumors by the World Health Organization (WHO) [1]. First described as pathologically distinct entities in 1945, subependymomas have a predilection for the fourth (50-60%) and lateral (30-40%) ventricles [2,3]. Characteristically indolent and asymptomatic, most go unreported; however, some studies estimate subependymomas to represent 0.2%–0.7% of all intracranial tumors and approximately 8% of ependymal tumors [4,5]. Rare clinical manifestations of subependymomas include hydrocephalus secondary to cerebrospinal fluid (CSF) obstruction and less frequently mass effect [6,7]. More commonly, subependymomas are clinically silent and treated with expectant management until hydrocephalus or mass effect warrants surgical resection [6].
On the other hand, ependymomas are WHO grade II tumors of neuroectodermal origin that constitute between 2%–6% of adult intracranial tumors and 6% - 10% of pediatric brain tumors [8-11]. Ependymomas arise within the ventricular system of the central neuraxis and are most commonly infratentorial (60%), showing a predilection for the floor of the fourth ventricle [12]. Given the increased risk of recurrence and drop metastasis associated with these tumors, complete surgical resection followed by postoperative radiation therapy represents the standard of care for ependymomas [13-17]. The significant difference in ependymoma and subependymoma behavior and thus management makes accurate preoperative diagnosis especially crucial. Considering both tumors arise in similar locations, magnetic resonance (MR) imaging characteristics can help differentiate the two diagnoses and guide management. Although both ependymomas and subependymomas are iso/ hypointense on T1 sequences and hyperintense on T2 relative to brain parenchyma, the major difference is that ependymomas exhibit heterogeneous patterns of enhancement, whereas subependymomas do not enhance at all [18]. Additionally, ependymomas tend to present in the posterior fossa and extend out of the foramina of Luschka (15%) or through the foramen of Magendie (60%), which subependymomas rarely do [19].
Remarkably, these rare tumors can present as a mixed lesion – that is, 5% to 20% of subependymomas harbor ependymomatous foci [20]. Combined subependymoma-ependymoma tumors behave more aggressively due in large part to the infiltrative nature of the ependymomatous component, thus underscoring the importance of understanding this entity and when to offer surgery [5]. Furthermore, the coincident presentation of these two tumors is a suggestive addition to the literature for a mixed tumor whose origin is currently unclear. In this report, we present an unusual case of a combined subependymoma-ependymoma in a 61-year old male and discuss the work-up, management, and prevailing pathogenesis of this mixed entity.
Case Report
History and examination
A 61-year-old ambidextrous handed male presented with headache and left hand tremor, and subsequently underwent intracranial MR imaging. He was diagnosed with a presumed subependymoma of the fourth ventricle and was managed expectantly by an outside hospital. Given continued symptoms, he sought a second opinion at our institution. The patient’s neurological examination was unremarkable, with the exception of a seemingly unrelated left hand tremor. Imaging demonstrated a 2 cm lesion centered within the fourth ventricle, with a focal area of contrast enhancement within the tumor, extending out the left foramen of Luschka with no concomitant hydrocephalus (Figure 1). While the lesion was initially felt to represent a subependymoma given its stability at 3 months on imaging, evidence of enhancement and growth into the left foramen of Luschka led to concern for the possible diagnosis of ependymoma and the decision was made to surgically resect the tumor for definitive diagnosis and treatment.
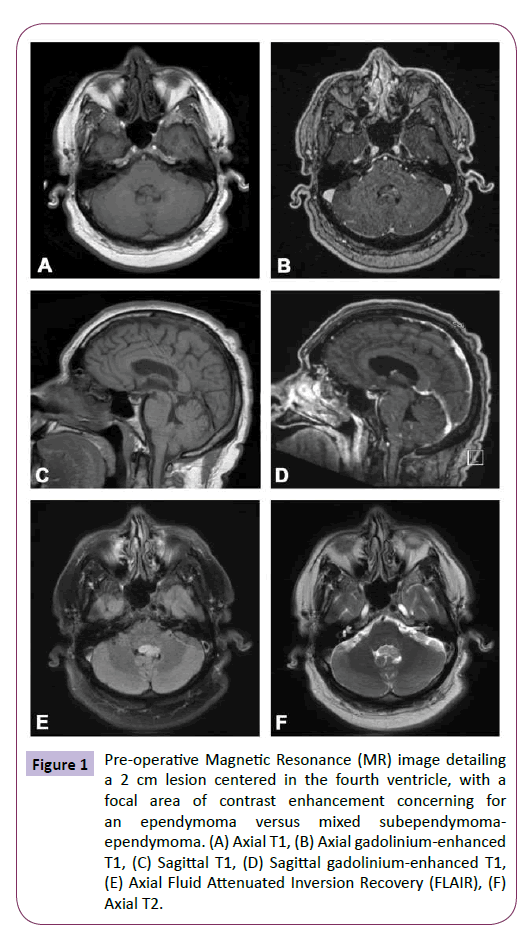
Figure 1: Pre-operative Magnetic Resonance (MR) image detailing a 2 cm lesion centered in the fourth ventricle, with a focal area of contrast enhancement concerning for an ependymoma versus mixed subependymomaependymoma. (A) Axial T1, (B) Axial gadolinium-enhanced T1, (C) Sagittal T1, (D) Sagittal gadolinium-enhanced T1, (E) Axial Fluid Attenuated Inversion Recovery (FLAIR), (F) Axial T2.
Operation
The patient underwent an uncomplicated suboccipital craniotectomy for resection of the fourth ventricular tumor with the aid of neuronavigation and neuromonitoring. Both sensory and motor evoked potentials, as well as lower cranial nerves (VII, IX, X, and XI) were monitored. An incision was extended from the inion of the external occipital protuberance down to C1 in the midline, and a standard craniectomy was performed to expose the posterior fossa. The cisterna magna was opened, allowing for CSF release and brain relaxation. An arachnoid plane between the cerebellar tonsils was identified and opened sharply, allowing for entrance into the fourth ventricle, and the vermis was elevated to allow for further tumor visualization. On gross inspection, the tumor was noted to be heterogeneous in appearance, similar in texture and color to the cerebellum in some areas, but blue and hemorrhagic in the lateral aspect of the tumor, which extended out Luschka. A plane between the tumor and the floor of the fourth ventricle was maintained, but became more difficult near the left foramen of Luschka as the tumor appeared more infiltrative in this location. Nonetheless, gross total resection (GTR) was achieved.
Pathological findings
Pathological examination confirmed the diagnosis of a mixed subependymoma-ependymoma. Histological analysis revealed a densely cellular tumor with uniform nuclei and perivascular ependymal pseudorosettes, diagnostic for WHO grade II ependymoma [21]. The tumor cells were positive for GFAP and negative for p53 and IDH1. Mitoses were rare and the Ki- 67 was low and estimated at less than 3%. There were also microcalcifications and focally hyalinized vascular channels. Closer histological examination demonstrated that it was subependymoma with an ependymomatous portion (Figure 2).
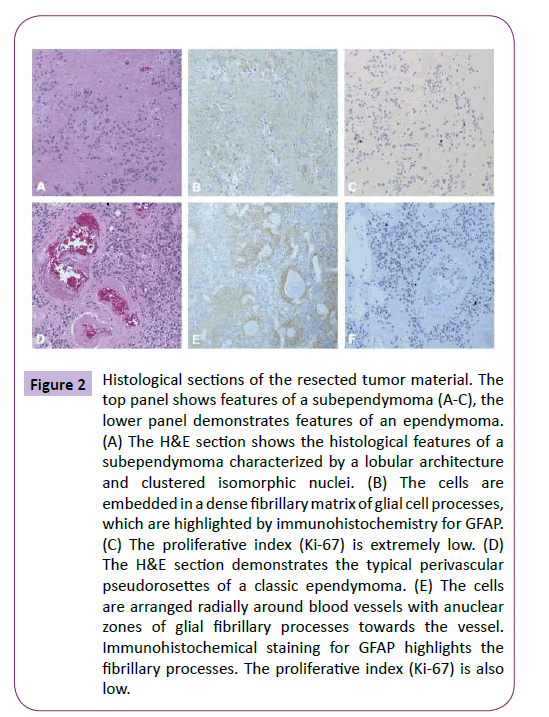
Figure 2: Histological sections of the resected tumor material. The top panel shows features of a subependymoma (A-C), the lower panel demonstrates features of an ependymoma. (A) The H&E section shows the histological features of a subependymoma characterized by a lobular architecture and clustered isomorphic nuclei. (B) The cells are embedded in a dense fibrillary matrix of glial cell processes, which are highlighted by immunohistochemistry for GFAP. (C) The proliferative index (Ki-67) is extremely low. (D) The H&E section demonstrates the typical perivascular pseudorosettes of a classic ependymoma. (E) The cells are arranged radially around blood vessels with anuclear zones of glial fibrillary processes towards the vessel. Immunohistochemical staining for GFAP highlights the fibrillary processes. The proliferative index (Ki-67) is also low.
Postoperative course
The patient tolerated surgery well and was discharged home on postoperative day 3, neurologically intact. His tremor remained and indeed appeared to be incidental and unrelated. An MRI of the spine was performed and did not demonstrate any “drop” metastases common to ependymomas. Although GTR was achieved, the infiltrative nature of the tumor led to the concern for residual microscopic disease and thus a decision was made by our multidisciplinary tumor board to treat the patient with adjuvant radiotherapy. Specifically, the patient underwent 54 gray (Gy) of fractionated External Beam Radiation Therapy (EBRT) over 6 weeks. He continues to do well and is being followed with surveillance imaging (Figure 3).
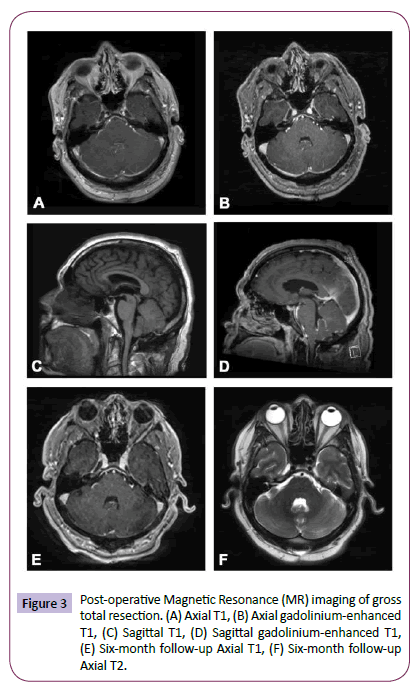
Figure 3: Post-operative Magnetic Resonance (MR) imaging of gross total resection. (A) Axial T1, (B) Axial gadolinium-enhanced T1, (C) Sagittal T1, (D) Sagittal gadolinium-enhanced T1, (E) Six-month follow-up Axial T1, (F) Six-month follow-up Axial T2.
Discussion
We report a mixed subependymoma-ependymoma entity that offers an important teaching point on the sometimes-coincident presentation of these two tumors. Accurate diagnosis of a combined subependymoma-ependymoma is especially important because the two entities are treated very differently. Standard treatment for subependymomas is expectant management given their typically benign clinical course, unless rare clinical manifestations of hydrocephalus or focal mass effect necessitate surgery. On the other hand, the treatment for ependymoma is surgical given the expectation for future growth and invasion, with residual disease on postoperative imaging being the most important prognostic variable [22]. Additionally, postoperative adjuvant therapy is usually prescribed as standard of care for ependymomas [23]. Given subependymomas can present with ependymomatous foci and subsequently require more aggressive treatment, practitioners must be perceptive to concerning features, such as contrast enhancement on imaging, extension into foramina, and invasion of surrounding tissue, that warrant surgical resection.
Unfortunately, the data on mixed-subependymomas are limited; a review of the current English literature in PubMed yields three studies that describe mixed pathology subependymomas (Table 1) [21,24,25]. Arvanitis et al. describe the case of a 40-year-old male presenting with a one-year history of intermittent headache and vertigo. MRI revealed a heterogeneously enhancing 2 cm mass with cystic and calcific components in the anterior horn of the left lateral ventricle. The tumor was partially resected through a left frontal craniotomy with transfrontal approach to the left ventricle, with histology revealing a combined tanycytic ependymoma and subependymoma.
| Number of Cases |
Reference |
Presentation |
Pathology |
Site |
Size (cm) |
Age (years) |
Sex |
Treatment |
Follow-up and Outcome |
Tumor Markers |
| 1 |
Present Case |
Headache and left hand tremor |
Mixed subependymoma - ependymoma (WHO Grade II) |
Fourth ventricle with extension into the foramen of Luschka |
2.0 |
61 |
Male |
Gross Total Surgical Resection and 54 gray (Gy) of fractionated External Beam Radiation Therapy (EBRT) over 6 weeks |
No signs of recurrence at 13 month follow-up |
GFAP +; p53 -; IDH1 -; Ki67 low proliferative index |
| 1 |
[24] |
One year history of vertigo and intermittent headaches |
Combined tanycyticependymoma and subependymoma (WHO Grade II) |
Anterior horn of the left lateral ventricle |
2.5 |
40 |
Male |
Partial resection through a left frontal craniotomy with transfrontal approach to the left ventricle |
No signs of recurrence |
GFAP +; S-100 +; EMA -; Ki67 low proliferative index |
| 1 |
[25] |
Gait ataxia, obstructive hydrocephalus, and cerebellar signs 6 years after surgical resection of a fourth ventricular subependymoma (WHO Grade I) |
Subependymoma with atypical features |
Fourth ventricle with invasion into the surround cerebellar parenchyma |
2.5 |
62 |
Female |
Surgical resection of tumor recurrence |
Post-operative course complicated by multiple supratentorial infarcts. Patient died 3 months after surgery due to fourth ventricular hemorrhage with mass effect. |
GFAP +; p53 +; EMA -; MIB-1 labeling index was high at up to 15% in some areas |
| 8 |
[21] |
Not given |
Mixed subependymoma - ependymoma (WHO Grade II) |
Foramen of Monroe (4); Lateral Ventricle (2); Fourth Ventricle (2) |
4 - 6 |
27 |
Female |
Subtotal surgical resection and Radiation Therapy |
Improved patient outcome (5); Worsened patient outcome (2); Death (1) |
Not given |
Table 1: Review of previously reported subependymomas with mixed features including the present case.
Zhiyong et al. present 43 cases of intracranial subependymomas treated at Beijing Tiantan Hospital between 2003 and 2013, 8 of which were pathologically confirmed to harbor WHO grade II ependymomatous foci. Among the 43 patients with subependymomas, all underwent gross total or partial surgical resection and two received adjuvant radiotherapy. Radiological imaging revealed tumors measuring 4 to 6 cm, with 4 lesions located near the Foramen of Monroe, 2 in the lateral ventricle, and 2 in the fourth ventricle. The authors found that in younger patients, subependymomas tended to be mixed infratentorial tumors while in patients 14 years of age or older, the lesions tended to be pure supratentorial subependymomas.
Tiwari et al. report a 62-year-old woman presenting with recurrent symptoms of gait ataxia and cerebellar signs 6 years following gross total resection of a fourth ventricular subependymoma. MRI revealed an enhancing fourth ventricular mass with likely invasion into the cerebellar parenchyma. The patient underwent surgical resection of the recurrent tumor and postoperatively, suffered obstructive hydrocephalus, lower cranial nerve deficits, and severe vasospasms that resulted in multiple supratentorial infarcts. The patient died of fourth ventricular hemorrhage with mass effect 3 months following surgery. Histological examination of the lesion confirmed a 2.4 cm subependymoma with atypical features, such as a high MIB-1 labeling index, more characteristic of ependymomas. The absence of such features in the primary occurrence of the patient’s tumor 6 years prior led the authors to conclude that this phenomenon either indicated a missed secondary component of the original tumor (potentially an ependymomatous component) that underwent high-grade transformation or, although never demonstrated as part of the natural history of this tumor, represented an anaplastic transformation of a WHO grade I subependymoma.
Together, these reports present 11 previous cases of mixed pathology subependymomas, demonstrating the paucity of these tumors. The typical size at presentation ranged from 2 to 6 cm, and although they can occur anywhere in the ventricular system, the majority (6 of the 11 cases) showed a predilection for the posterior fossa. Given the ependymal component, all tumors were managed with gross total or subtotal surgical resection, while only two received adjuvant radiation therapy.
The similarity of their names belies their differing origins; ependymomas are generally accepted to arise from radial glia while the histogenesis of subependymomas remains unknown [26]. Candidate precursors include astrocytes of the subependymal plate, subependymal glia, ependymal cells, tanycytes, or some combination of these cells [27,28]. Some investigators even question whether subependymomas are conclusively neoplasms, arguing that subependymomas represent a local maldevelopment, or hamartoma, pointing to concurrent heterotopic leptomeningeal neuroglial tissue [29] and cellular resemblance to mature subependymal tissue [30] as evidence. Others maintain that subependymomas are genuine neoplastic lesions, citing their potential growth and facility to recur [31].
Early cases of concurrent tumors in twins led researchers to posit that subependymomas originated from a prenatal subpopulation of cells [32,33]. Other theories proposed early postnatal gliogenesis, where subependymal stem cells give rise to glial progenitor cells, which then migrate to outer peripheral zones [34]. However, later examination of spinal ependymomas contradicted this theory because a subependymal zone glial origin could not explain their exophytic and predominantly peripheral localization. As a consequence, Horner et al. demonstrated two alternatives more consistent with contemporary data where cell proliferation is primarily located in the outer zones of the spinal cord [35]. The first theory posits that an asymmetrically dividing stem cell gives rise to a daughter glial progenitor cell that migrates to the periphery and multiplies. The second proposes that these multipotent stem cells already exist in the outer zone of the central cord and subsequently produce glial progenitors.
The concurrent occurrence of both a subependymoma and ependymoma seen in our patient is a rare phenomenon of unknown origin. Three main mechanisms have been proposed for the origin of tumors exhibiting mixed histologic characteristics: the first posits the “collision” of two distinct histologic clones, the second describes a “combination” phenomenon where each neoplastic element is derived from a common progenitor stem cell, and the third ventures a “conversion” hypothesis where genetic instability in one tumor causes spontaneous differentiation into the other [24,25]. The dearth of literature on the topic of mixed subependymoma-ependymomas, albeit consistent with the rarity of these lesions, means insufficient data exists to definitively support one theory over another.
Some investigators argue that these mixed tumors are the result of a shared progenitor [21], pointing to ultrastructural observation of ependymal structures in these lesions as evidence suggesting subependymomas are variants of ependymomas [36-39]. While this may hold some weight, we believe that the collision of two distinct neoplastic clones, a well-documented phenomenon in other mixed tumors [40-46], is more likely given that subependymomas exhibit distinct microscopic and pathologic features-for example, they lack the tight junctional complexes of ependymomal microrosettes [47]. There are various hypotheses that postulate the pathogenesis of collision tumors: the occurrence of two distinct neoplasms is purely coincidental; the shared site is conducive to the occurrence of multiple tumors due to an environmental carcinogenic stimulus; or the presence of a primary tumor creates a microenvironment favorable to the development of a second tumor [48]. Future research and gene sequencing of such mixed entities may begin to answer the difficult pathological question of combined subependymomaependymoma origin.
Conclusions
Mixed subependymoma-ependymoma tumors are rare clinical entities that have the potential for misdiagnosis. Contrast enhancement, albeit faint at times, and growth into surrounding structures can facilitate the diagnosis of a mixed or pure ependymomatous lesion and subsequent initiation of more aggressive management. Although the simultaneous occurrence of these tumors contributes to the literature on whether these mixed entities represent a coincident presentation of two primary malignancies, a shared progenitor, or one tumor dedifferentiating into the other, the histogenesis of mixed subependymomaependymomas remains controversial. Future research and gene sequencing should provide a clearer understanding of the precise origin and pathogenesis of these mixed lesions. Regardless, we employ clinicians to have a low index of suspicion for mixed subependymoma-ependymoma lesions, as misdiagnosis can bring great harm.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this case report or the findings specified in this paper.
7178
References
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, et al. (2007) The 2007 WHO classification of tumours of the central nervous system. Actaneuropathologica 114:97-109.
- Scheinker M (1945)Subependymoma: a newly recognized tumor of subependymal derivation. Journal of neurosurgery 2:232-240.
- Jain A, Amin AG, Jain P, Burger P, Jallo GI, et al. (2012) Subependymoma: clinical features and surgical outcomes. Neurological research 34:677-684.
- Ragel BT, Osborn AG, Whang K, Townsend JJ, Jensen RL, et al. (2006) Subependymomas: an analysis of clinical and imaging features. Neurosurgery 58:881-890.
- Scheithauer BW (1978) Symptomatic subependymoma. Report of 21 cases with review of the literature. Journal of neurosurgery 49:689-696.
- Rushing EJ, Cooper PB, Quezado M, Begnami M, Crespo A, et al. (2007) Subependymoma revisited: clinicopathological evaluation of 83 cases. Journal of neuro-oncology 85:297-305.
- Lobato RD, Sarabia M, Castro S, Esparza J, Cordobes F, et al. (1986) Symptomatic subependymoma: report of four new cases studied with computed tomography and review of the literature. Neurosurgery 19:594-598.
- Rawlings CE, Giangaspero F, Burger PC, Bullard DE (1988)Ependymomas: a clinicopathologic study. Surgical neurology 29:271-281.
- Reni M, Gatta G, Mazza E, Vecht C (2007)Ependymoma. Critical reviews in oncology/hematology 63:81-89.
- Barone BM, Elvidge AR (1970)Ependymomas. A clinical survey. Journal of neurosurgery 33:428-438.
- Goldwein JW, Leahy JM, Packer RJ, Sutton LN, Curran WJ, et al. (1990) Intracranial ependymomas in children. International journal of radiation oncology, biology, physics 19:1497-1502.
- McLaughlin MP, Marcus RB, Buatti JM, McCollough WM, Mickle JP, et al. (1998) Ependymoma: results, prognostic factors and treatment recommendations. International journal of radiation oncology, biology, physics 40:845-850.
- Bloom HJ, Glees J, Bell J, Ashley SE, Gorman C (1990)The treatment and long-term prognosis of children with intracranial tumors: a study of 610 cases, 1950-1981. International Journal of Radiation OncolBiolPhys18:723-745.
- Salazar OM, Castro VH, VanHoutte P, Rubin P, Aygun C (1983) Improved survival in cases of intracranial ependymoma after radiation therapy. Late report and recommendations. Journal of neurosurgery 59:652-659.
- Di Marco A, Campostrini F, Pradella R, Reggio M, Palazzi M, et al. (1988) Postoperative irradiation of brain ependymomas. Analysis of 33 cases. Actaoncologica (Stockholm, Sweden)27:261-267.
- Aizer AA, Ancukiewicz M, Nguyen PL, Macdonald SM, Yock TI, et al. (2013) Natural history and role of radiation in patients with supratentorial and infratentorial WHO grade II ependymomas: results from a population-based study. Journal of neuro-oncology 115:411-419.
- Cage TA, Clark AJ, Aranda D, Gupta N, Sun PP, et al. (2013) A systematic review of treatment outcomes in pediatric patients with intracranial ependymomas. Journal of neurosurgery Pediatrics 11:673-681.
- Smith AB,Smirniotopoulos JG, HorkanyneSI (2013)From the radiologic pathology archives: intraventricular neoplasms: radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc 33:21-43.
- Yuh EL, Barkovich AJ, Gupta N (2009) Imaging of ependymomas: MRI and CT. Child's nervous system :ChNS : official journal of the International Society for Pediatric Neurosurgery 25:1203-1213.
- Perry A, Brat DJ (2010) Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition Series: Elsevier Health Sciences.
- Zhiyong Bi, XiaohuiRen, Junting Zhang, Wang Jia (2015) Clinical, radiological, and pathological features in 43 cases of intracranial subependymoma. Journal of neurosurgery 122:49-60.
- Healey EA, Barnes PD, Kupsky WJ, Scott RM, Sallan SE, et al. (1991) The prognostic significance of postoperative residual tumor in ependymoma. Neurosurgery 28:666-671.
- Metellus P, Guyotat J, Chinot O, Durand A, Barrie M, et al. (2010) Adult intracranial WHO grade II ependymomas: long-term outcome and prognostic factor analysis in a series of 114 patients. Neuro-oncology 12:976-984.
- Arvanitis LD, Gattuso P, Nag S (2013) A 40-year-old male with an intraventricular tumor. Combined tanycyticependymoma and subependymoma. Brain Pathology 23:359-260.
- Tiwari N, Powell SZ, Takei H (2015) Recurrent subependymoma of fourth ventricle with unusual atypical histological features: A case report. Pathology international.
- Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, et al. (2005) Radial glia cells are candidate stem cells of ependymoma. Cancer cell 8:323-335.
- Prayson RA (2011) Neuropathology: A Volume in the Series: Foundations in Diagnostic Pathology: Elsevier Health Sciences.
- Maekawa M, Fujisawa H, Iwayama Y, Tamase A, Toyota T, et al. (2010) Giant subependymoma developed in a patient with aniridia: analyses of PAX6 and tumor-relevant genes. Brain pathology (Zurich, Switzerland) 20:1033-1041.
- Ho KL (1983) Concurrence of subependymoma and heterotopic leptomeningealneuroglial tissue. Archives of pathology & laboratory medicine 107:136-140.
- Azzarelli B, Rekate HL, Roessmann U (1977)Subependymoma: a case report with ultrastructural study. Actaneuropathologica 40:279-282.
- Shimada S, Ishizawa K, Horiguchi H, Shimada T, Hirose T (2003)Subependymoma of the spinal cord and review of the literature. Pathology international 53:169-173.
- Clarenbach P, Kleihues P, Metzel E, Dichgans J (1979) Simultaneous clinical manifestation of subependymoma of the fourth ventricle in identical twins. Case report. Journal of neurosurgery 50:655-659.
- Noell S, Beschorner R, Bisdas S, Beyer U, Weber RG, et al. (2014) Simultaneous subependymomas in monozygotic female twins: further evidence for a common genetic or developmental disorder background. Journal of neurosurgery.
- Krishnan SS, Panigrahi M, Pendyala S, Rao SI, Varma DR (2012) Cervical Subependymoma: A rare case report with possible histogenesis. Journal of neurosciences in rural practice 3:366-369.
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, et al. (2000) Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. The Journal of neuroscience: the official journal of the Society for Neuroscience 20:2218-2228.
- Russell DSR, Rubinstein LJ (1971) Pathology of Tumours of the Nervous System. 3rd ed. Baltimore: Williams and Wilkins.
- Rubinstein LJ (1972) Tumors of the central nervous system.Atlas of Tumor Pathology. 2nd ed. Washington, DC: Armed Forces Institute of Pathology.
- Zulch D, Rothballer AB, Olezewski J (1965) Brain Tumors, Their Biology and Pathology (2nd edn) New York: Springer.
- Fu YS, Chen AT, Kay S, Young H (1974)Issubependymoma (subependymalglomerate astrocytoma) an astrocytoma or ependymoma? A comparative ultrastructural and tissue culture study. Cancer 34:1992-2008.
- Jin G, Hao S, Xie J, Mi R, Liu F (2013) Collision tumors of the sella: coexistence of pituitary adenoma and craniopharyngioma in the sellar region. World journal of surgical oncology 11:178.
- Takahashi FJ, Matumoto M, Kan I, Oka H, Yasue M (2012) Atypical teratoid/rhabdoid tumor with 26-year overall survival: case report. Journal of neurosurgery Pediatrics 9:400-405.
- Molnar P, Hegedus K (1984)Adjacent astrocytoma and ependymoma: dependent mixed neoplasia or unusual collision tumors? Surgical neurology 22:455-460.
- Brahmania M, Kanthan CS, Kanthan R (2007) Collision tumor of the colon--colonic adenocarcinoma and ovarian granulosa cell tumor. World journal of surgical oncology 5:118.
- Kleist B, Lasota J, Miettinen M (2010)Gastrointestinal stromal tumor and gastric adenocarcinoma collision tumors. Human pathology 41:1034-1039.
- Tippu SR, Rahman F, Sharma N, Srivastava S (2014) Collision tumor of the palate: A rare case report. Contemporary clinical dentistry 5:102-105.
- Sughayer MA, Zakarneh L, Abu-Shakra R (2009) Collision metastasis of breast and ovarian adenocarcinoma in axillary lymph nodes: a case report and review of the literature. Pathology oncology research: POR 15:423-427.
- Moss TH (1984) Observations on the nature of subependymoma: an electron microscopic study. Neuropathology and applied neurobiology 10:63-75.
- BrandweinGM, Urken M, Wang B (2004) Collision tumor of the thyroid: a case report of metastatic liposarcoma plus papillary thyroid carcinoma. Head & neck 26:637-641.








