Case report: ‘Photodynamics of Subependymal Giant Cell Astrocytoma with 5-Aminolevulinic acid’
- 1King's Neuro Lab, Department of Neurosurgery, London, United Kingdom
- 2School of Biomedical Engineering and Imaging Studies, King's College London, London, United Kingdom
- 3Department of Neuropathology, King's College Hospital NHS Foundation Trust, London, United Kingdom
- 4Department of Neurosurgery, King's College Hospital NHS Foundation Trust, London, United Kingdom
Subependymal Giant Cell Astrocytoma (SEGA) is a common diagnosis in patients with Tuberous Sclerosis. Although surgical treatment is often required, resection may entail a significant risk for cognitive function given the anatomical relation with critical structures such as the fornices and subgenual area. Therefore, target subtotal resections using minimal invasive approaches focused in the higher metabolic areas are valuable options to preserve quality of life while addressing specific problems caused by the tumor, such as hydrocephalus or progressive growth of a specific component of the tumor. In this report, the authors explore the potential role of 5-ALA in the identification of highly metabolic areas during SEGA resection in the context of minimal invasive approaches.
Introduction
Subependymal Giant Cell Astrocytoma (SEGA) is a low-grade tumor (WHO Grade I) (1) predominantly associated with tuberous sclerosis (2–4). It characteristically arises from the wall of lateral ventricles, and while they are often asymptomatic, symptoms and neurological deterioration can occur as a result of obstructive hydrocephalus.
On imaging, SEGA classically appears as a paraventricular mass near the foramen of Monro, larger than 1 cm, showing calcifications, heterogeneous MRI signal, and marked contrast enhancement. Surgical resection is usually curative for such tumors except when there are multiple tumors in which case medical treatment is favored (5–8).
The value of 5-aminolevulinic acid (5-ALA) for resection of high grade or malignant astrocytomas has been established in recent years (9, 10). However, its use remains controversial in the context of low-grade gliomas, with limited data available (11). While the actual mechanism for 5-ALA uptake by the cells is not completely understood, disturbances in the heme group synthesis pathway, disturbances in the blood-brain barrier, and increase aquaporin expression have all been implicated, although there remains a lack of evidence for a single or driving mechanism (12).
In this report, the authors report the usefulness of 5-ALA assisted resection in a SEGA and perform a review of the existing literature.
Case description
A 32 years old lady, with a background of tuberous sclerosis, was referred to the neuro-oncology service with an incidental finding of SEGA during investigation for migraine (Figure 1). She presented with worsening headaches secondary to obstructive hydrocephalus, which was successfully treated with the insertion of a Ventriculo-Peritoneal shunt (VPS). She remained under surveillance with regular imaging for the underlying tumor. Imaging at 2 years revealed mild tumor growth with obstruction of the foramen of Monro, enlargement of the cystic component of the tumor, and entrapment of the ipsilateral ventricle. Surgical resection was therefore advised after multidisciplinary team discussion.
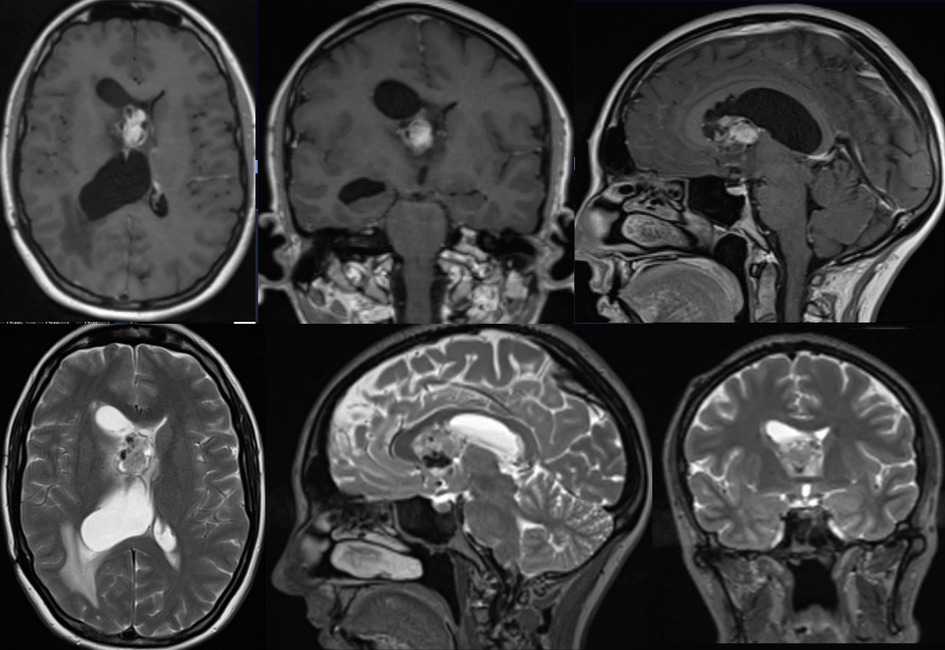
Figure 1. Pre-op MRI scan with T1 post gad and T2 weighted images showing right sided heterogeneous intraventricular lesion with low T2 signal likely calcified components with some areas of avid gadolinium enhancement.
Surgical resection was performed with pre-operative tractography and mapping (Figures 2A,B) A transsulcal minimally invasive parafascicular approach (tsMIPS) was carried out using NICO BrainPath system© with assistance of neuronavigation, 5-ALA, intraoperative neurophysiological monitoring (IONM), and intraoperative ultrasound. Intraoperative transdural mapping of the primary motor cortex confirmed the cortical location of both upper and lower limbs.
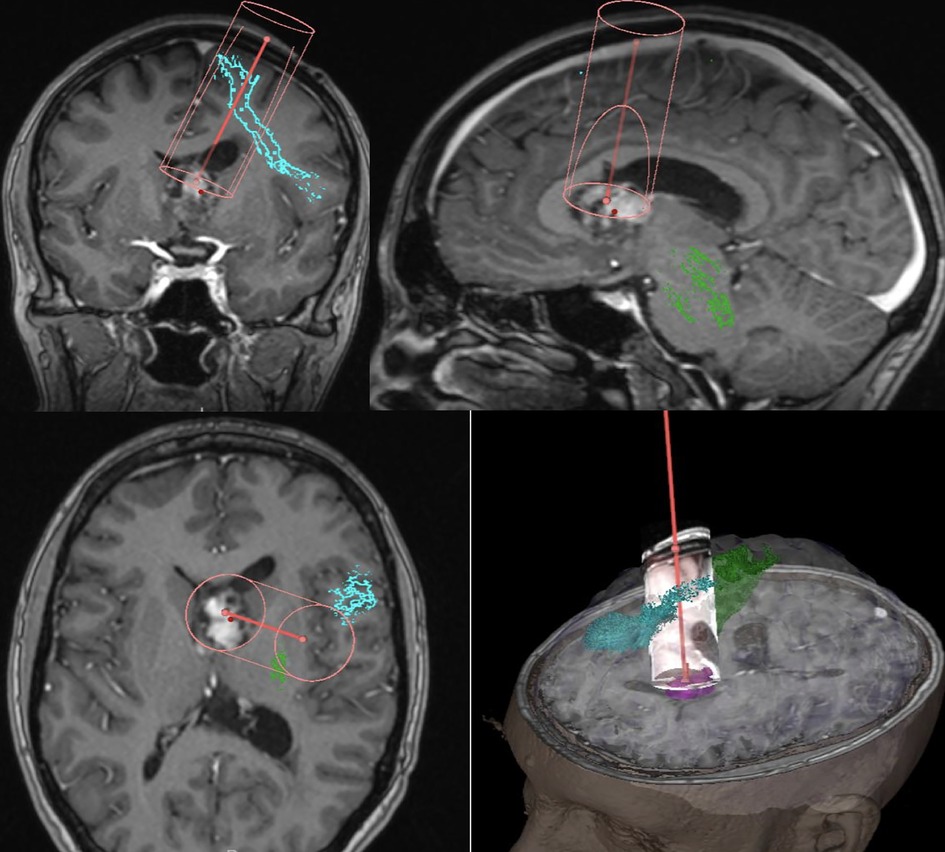
Figure 2. Pre-operative mapping and trajectory planning, Green – CST (cortico spinal tract), Blue – FAT, Magenta – Tumor.
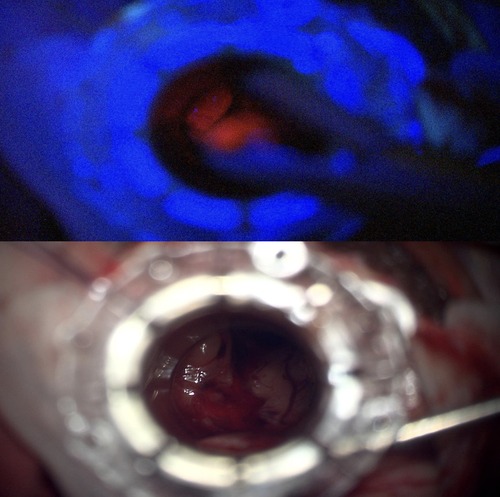
Intraoperatively, the tumor was found to have both bright and pale fluorescence under Blue 400 filter—Zeiss Pentero 900 (Carl Zeiss Meditec®), in the cystic and solid components respectively (Figures 3A,B). A decision was made to limit the resection to the 5-ALA positive tissue and the solid calcified component leaving tumor attached to the walls of fornix, walls of the third ventricle and hypothalamus. No fluorescence was perceived at the end of resection (Figure 4).
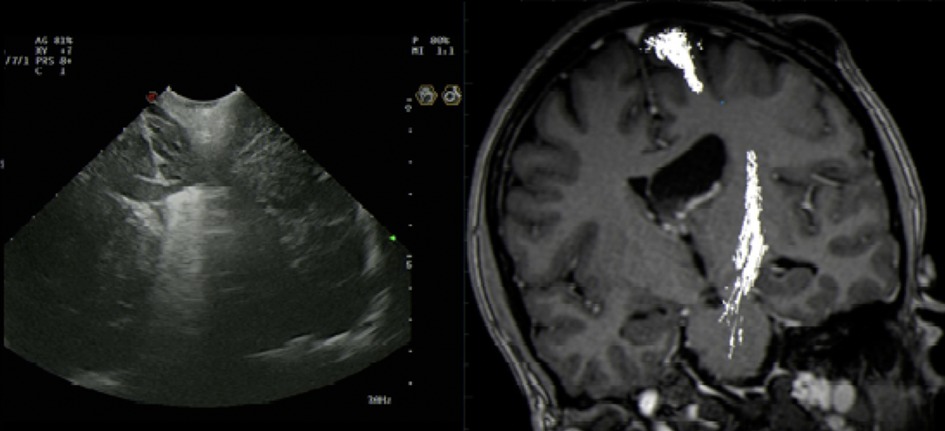
Figure 4. Intraoperative US artefact caused by Residual intraventricular blood and calcified component.
The patient made a good recovery with no neurological deficits and post-operative imaging confirmed no complications or evidence of ventriculomegaly and resolution of the entrapped lateral ventricle (Figure 5).
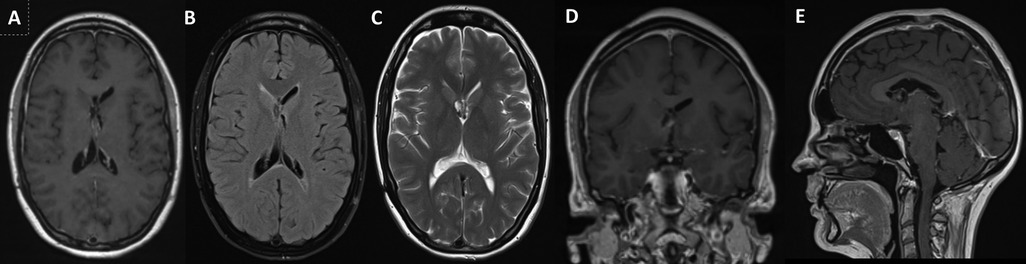
Figure 5. 3-Year Follow-up Postoperative MRI – Axial (A) – T1-Gad weighted, (B) – FLAIR and (C) – T2 weighted, Coronal (D) - T1-Gad weighted and Sagittal (E) - T1-Gad weighted) images showing near total resection with minor contrast enhancing in the lateral walls of the third ventricle and in the fornices. This is the last follow-up scan and the patient has not received further treatment apart from surgery.
Histopathology confirmed Subependymal Giant Cell Astrocytoma (SEGA, WHO grade I). (Figure 6) No adjuvant treatment was required and the patient was under follow-up of neuro-oncology multidisciplinary team with last follow up three years after surgery with stable residual component and no symptoms reported.
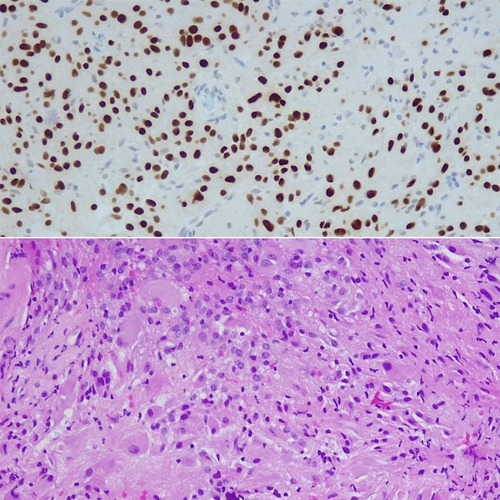
Figure 6. Histology- Areas of epithelioid appearances with large vesicular nuclear and prominent nucleoli associated with abundant granular or ground glass cytoplasm, merged with spindle-shaped cells forming compact glial fibrillary background. Focal calcification visible, tumor also attached to ependymal cells.
Discussion
SEGA is a rare tumor and appears approximately in 20%–27.4% (4) of patients with tuberous sclerosis. Increase in seizure frequency (15.8%), behavioral disturbance (11.9%), regression/loss of cognitive skills (9.9%) were identified as frequent symptoms associated with SEGA, over and above headaches (8.4%), typically associated with raised intracranial pressure (13).
Extent of tumor resection with no neurological deficits can be technically difficult due to the location and adherence to surrounding tissue and the nature of the tumor, which is usually calcified (14). As with the majority of cases, the tumor in our patient was located in periventricular region, in proximity to the foramen of Monro, and close to subgenual area and the fornix. This made it equally important and challenging for safe resection without damaging important equivocal areas as resection of these lesions can be associated with significant complications particularly in larger lesions – up to two thirds in lesions larger than 3 cm (5). Therefore, a decision towards a fluorescence-guided resection of the higher-grade component was taken to preserve patient's quality of life.
5-ALA was used in our case to target the surgical removal as subtotal resection was planned to preserve the patient's cognition and neurophysiological function. Fluorescence for low grade tumors has been reported with variable results - 8% (15) 9% (16) 16% (the largest published series) (17) 33% (18) and 40% (19) and therefore difficult to generalize. In addition, some benign lesions have been recently reported by our colleagues as fluorescent with 5-ALA, particularly endodermal cysts and glioneuronal tumors (20). Recently, we have successfully presented this case report as a poster at British Neuro-Oncology Society (BNOS) conference in Liverpool (21).
To the best of our knowledge, there have been only two case reports published so far which includes a total of 3 cases mentioning the use of 5-ALA (Gliolan®) in the resection of SEGA (22, 23) (Table 1). Interestingly, two out of three were reported as bright on fluorescence whereas the remaining one was pale. The extent of resection was reported as gross total resection (GTR) for one case whereas there was no data for the rest of two cases. The case with GTR was reported as having no post op complications.
There was limited data to report any variation in fluorescence brightness within the tumor, which we aim to report here. In our case, we found that the soft or cystic part of the tumor showed higher fluorescence compared to the calcified part. Metabolic imaging was not available for this patient. Further research should focus on the relation between intraoperative 5-ALA fluorescence and preoperative metabolic imaging, such as PET-FGD or PET-amino acids.
The reasons for the low degree of fluorescence of the calcified component are not completely understood. There was a systematic review published in 2016 which evaluated the relationship between the 5-ALA fluorescence and tumor histopathology (24). In a study of Grade II and Grade III Gliomas, it was reported that 5-ALA is a reliable marker for tumor anaplasticity (sensitivity: 89%; specificity: 88%; ppv:85%; npv: 91%) (25). Further there was another study to support this which found that the intensity of fluorescence is representative of the degree of malignancy within the tumor (17). These findings of increased fluorescence rates in high grade gliomas compared to low grade allow us to reasonably conclude that fluorescence positivity is related to higher degree of malignancy. Hence by looking at these studies, we can hypothesize the reason for low uptake of 5-ALA (Gliolan®) in the calcified portion to be the area of low degree of malignancy.
In this case, 5-ALA guided-resection was not used to improve the extent of resection but to target specific areas within the tumor related with potentially higher proliferation areas given our knowledge of 5-ALA metabolism. Despite the use of a minimal invasive approach to resect this tumor, the extent of resection was not limited by this approach. Even if different surgical approaches have been used – such as transcallosal or transcortical, the decision to limit the extent of resection was based on the risk of neurological injury and not on lack of visualization, maneuverability or resectability.
The MIPS approach integrated with the preoperative planning provided by tractography and intraoperative neuromonitoring and mapping allowed for a safer resection and successful preservation of the patient's quality of life.
Conclusion
5-ALA-guided resection is a potential adjunct not only for maximizing surgical resection but also to target focal subtotal resections in eloquent areas. Its use in SEGA has been rarely reported. Therefore, further studies in the area of photodynamics will be helpful to fully evaluate the efficacy of 5-ALA in SEGA and other low-grade tumors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
KA, FV, RB and JPL put forward the idea for this case report. IG wrote the draft of the manuscript and IG and SP did the literature review. SP, JPL and PG revised the draft and made corrections. Finally, All authors contributed to the article and approved the submitted version.
Conflict of interest
PG is a Review Editor for Neurosurgery at Frontiers in Surgery.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131(6):803–20. doi: 10.1007/s00401-016-1545-1
2. Jansen AC, Belousova E, Benedik MP, Carter T, Cottin V, Curatolo P, et al. Newly diagnosed and growing subependymal giant cell astrocytoma in adults with tuberous sclerosis Complex: results from the international TOSCA study. Front Neurol. (2019) 10:821. doi: 10.3389/fneur.2019.00821
3. Chan DL, Calder T, Lawson JA, Mowat D, Kennedy SE. The natural history of subependymal giant cell astrocytomas in tuberous sclerosis complex: a review. Rev Neurosci. (2018) 29(3):295–301. doi: 10.1515/revneuro-2017-0027
4. Adriaensen ME, Schaefer-Prokop CM, Stijnen T, Duyndam DA, Zonnenberg BA, Prokop M. Prevalence of subependymal giant cell tumors in patients with tuberous sclerosis and a review of the literature. Eur J Neurol. (2009) 16(6):691–6. doi: 10.1111/j.1468-1331.2009.02567.x
5. Kotulska K, Borkowska J, Roszkowski M, Mandera M, Daszkiewicz P, Drabik K, et al. Surgical treatment of subependymal giant cell astrocytoma in tuberous sclerosis complex patients. Pediatr Neurol. (2014) 50(4):307–12. doi: 10.1016/j.pediatrneurol.2013.12.004
6. Roth J, Roach ES, Bartels U, Jóźwiak S, Koenig MK, Weiner HL, et al. Subependymal giant cell astrocytoma: diagnosis, screening, and treatment. Recommendations from the international tuberous sclerosis Complex consensus conference 2012. Pediatr Neurol. (2013) 49(6):439–44. doi: 10.1016/j.pediatrneurol.2013.08.017
7. Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Belousova E, Frost MD, et al. Everolimus long-term use in patients with tuberous sclerosis complex: four-year update of the EXIST-2 study. PLoS One. (2017) 12(8):e0180939. doi: 10.1371/journal.pone.0180939
8. Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol. (2014) 15(13):1513–20. doi: 10.1016/S1470-2045(14)70489-9
9. Della Puppa A, Ciccarino P, Lombardi G, Rolma G, Cecchin D, Rossetto M. 5-Aminolevulinic Acid fluorescence in high grade glioma surgery: surgical outcome, intraoperative findings, and fluorescence patterns. Biomed Res Int. (2014) 2014:232561. doi: 10.1155/2014/232561
10. Baig Mirza A, Christodoulides I, Lavrador JP, Giamouriadis A, Vastani A, Boardman T, et al. 5-Aminolevulinic acid-guided resection improves the overall survival of patients with glioblastoma-a comparative cohort study of 343 patients. Neurooncol Adv. (2021) 3(1):vdab047. doi: 10.1093/noajnl/vdab047
11. Almekkawi AK, El Ahmadieh TY, Wu EM, Abunimer AM, Abi-Aad KR, Aoun SG, et al. The use of 5-aminolevulinic acid in low-grade glioma resection: a systematic review. Oper Neurosurg (Hagerstown). (2020) 19(1):1–8. doi: 10.1093/ons/opz336
12. Molina EJ S, Ardon H, Schroeteler J, Klingenhöfer M, Holling M, Wölfer J, et al. Aquaporin-4 in glioma and metastatic tissues harboring 5-aminolevulinic acid-induced porphyrin fluorescence. Clin Neurol Neurosurg. (2013) 115(10):2075–81. doi: 10.1016/j.clineuro.2013.07.016
13. Jansen AC, Belousova E, Benedik MP, Carter T, Cottin V, Curatolo P, et al. Clinical characteristics of subependymal giant cell astrocytoma in tuberous sclerosis Complex. Front Neurol. (2019) 10:705. doi: 10.3389/fneur.2019.00705
14. Mei GH, Liu XX, Zhou P, Shen M. Clinical and imaging features of subependymal giant cell astrocytoma: report of 20 cases. Chin Neurosurg Jl. (2017) 3:14. doi: 10.1186/s41016-017-0077-4
15. Bernal García LM, Cabezudo Artero JM, García Moreno R, Marcelo Zamorano MB, Mayoral Guisado C. Fluorescence guided resection with 5-aminolevulinic acid of a pilomyxoid astrocytoma of the third ventricle. Neurocirugia (Astur). (2017) 28(5):251–6. doi: 10.1016/j.neucir.2017.03.002
16. Widhalm G, Kiesel B, Woehrer A, Traub-Weidinger T, Preusser M, Marosi C, et al. 5-Aminolevulinic Acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS One. (2013) 8(10):e76988. doi: 10.1371/journal.pone.0076988
17. Jaber M, Wölfer J, Ewelt C, Holling M, Hasselblatt M, Niederstadt T, et al. The value of 5-aminolevulinic acid in low-grade gliomas and high-grade gliomas lacking glioblastoma imaging features: an analysis based on fluorescence, magnetic resonance imaging, 18F-fluoroethyl tyrosine positron emission tomography, and tumor molecular factors. Neurosurg. (2016) 78(3):401–11; discussion 411. doi: 10.1227/NEU.0000000000001020
18. Valdés PA, Jacobs V, Harris BT, Wilson BC, Leblond F, Paulsen KD, et al. Quantitative fluorescence using 5-aminolevulinic acid-induced protoporphyrin IX biomarker as a surgical adjunct in low-grade glioma surgery. J Neurosurg. (2015) 123(3):771–80. doi: 10.3171/2014.12.JNS14391
19. Marbacher S, Klinger E, Schwyzer L, Fischer I, Nevzati E, Diepers M, et al. Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus. (2014) 36(2):E10. doi: 10.3171/2013.12.FOCUS13464
20. Lavrador JP, Kandeel HS, Kalb A, Reisz Z, Al-Sarraj S, Gullan R, et al. 5-ALA Fluorescence in a WHO grade I papillary glioneuronal tumour: a case report. Acta Neurochir (Wien). (2020) 162(4):813–7. doi: 10.1007/s00701-020-04223-x
21. Ghani I, Patel S, Bodi I, Vergani F, Ashkan K, Bhangoo R, et al. Photodynamics of subependymal giant cell astrocytoma (SEGA) with 5-aminolevulinic acid (5-ALA/gliolan©). Neuro-Oncol. (2022) Volume 24(Issue Supplement_4):Page iv20. doi: 10.1093/neuonc/noac200.091
22. Utsuki S, Oka H, Fujii K. Intraoperative photodynamic diagnosis of brain tumors using 5-aminolevulinic acid. In: Abujamra, AL, editor. Diagnostic techniques and surgical management of brain tumors. London: IntechOpen (2011). doi: 10.5772/19788
23. Höhne J, Acerbi F, Falco J, Akçakaya MO, Schmidt NO, Kiris T, et al. Lighting up the tumor—fluorescein-guided resection of gangliogliomas. J Clin Med. (2020) 9(8):2405. doi: 10.3390/jcm9082405
24. Ferraro N, Barbarite E, Albert TR, Berchmans E, Shah AH, Bregy A, et al. The role of 5-aminolevulinic acid in brain tumor surgery: a systematic review. Neurosurg Rev. (2016) 39(4):545–55. doi: 10.1007/s10143-015-0695-2
Keywords: 5-aminolevulinic acid, fluorescence, tuberous sclerosis, subependymal giant cell astrocytoma, low grade glioma, minimal invasive parafascicular approach
Citation: Ghani I, Patel S, Ghimire P, Bodi I, Bhangoo R, Vergani F, Ashkan K and Lavrador JP (2023) Case report: ‘Photodynamics of Subependymal Giant Cell Astrocytoma with 5-Aminolevulinic acid’. Front. Surg. 9:1065979. doi: 10.3389/fsurg.2022.1065979
Received: 10 October 2022; Accepted: 2 December 2022;
Published: 6 January 2023.
Edited by:
Mark Preul, Barrow Neurological Institute (BNI), United StatesReviewed by:
Ignazio Gaspare Vetrano, IRCCS Carlo Besta Neurological Institute Foundation, ItalyMukesch Johannes Shah, University of Freiburg, Germany
© 2023 Ghani, Patel, Ghimire, Bodi, Bhangoo, Vergani, Ashkan and Lavrador. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prajwal Ghimire prajwal.1.ghimire@kcl.ac.uk
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
 Imran Ghani
Imran Ghani Sabina Patel
Sabina Patel Prajwal Ghimire
Prajwal Ghimire Istvan Bodi
Istvan Bodi Ranjeev Bhangoo4
Ranjeev Bhangoo4  Francesco Vergani
Francesco Vergani Keyoumars Ashkan
Keyoumars Ashkan Jose Pedro Lavrador
Jose Pedro Lavrador