Phytochemistry, health benefits, and food applications of sea buckthorn (Hippophae rhamnoides L.): A comprehensive review
- 1Key Laboratory of Molecular Pharmacology and Drug Evaluation (Yantai University), Ministry of Education, Collaborative Innovation Center of Advanced Drug Delivery System and Biotech Drugs in Universities of Shandong, School of Pharmacy, Yantai University, Yantai, China
- 2Department of Organic Chemistry, School of Pharmacy, Naval Medical University, Shanghai, China
- 3School of Pharmacy, Binzhou Medical University, Yantai, China
Sea buckthorn (Hippophae rhamnoides L.), an ancient miraculous plant, is of great interest because of its tenacity, richness in nutritional active substances, and biological activity. Sea buckthorn is a deciduous shrub or tree of the genus Hippophae in the family Elaeagnaceae. It is a pioneer tree species for soil improvement, wind and sand control, and soil and water conservation. Sea buckthorn contains many nutritional active components, such as vitamins, carotenoids, polyphenols, fatty acids, and phytosterols. Moreover, sea buckthorn has many health benefits, such as antioxidant, anticancer, anti-hyperlipidemic, anti-obesity, anti-inflammatory, antimicrobial, antiviral, dermatological, neuroprotective, and hepatoprotective activities. Sea buckthorn not only has great medicinal and therapeutic potential, but also is a promising economic plant. The potential of sea buckthorn in the human food industry has attracted the research interest of researchers and producers. The present review mainly summarizes the phytochemistry, nutrients, health benefits, and food applications of sea buckthorn. Overall, sea buckthorn is a dietary source of bioactive ingredients with the potential to be developed into functional foods or dietary supplements for the prevention and treatment of certain chronic diseases, which deserves further research.
Introduction
Sea buckthorn (Hippophae rhamnoides L.) is a deciduous shrub or tree that is also known as Siberian pineapple, sand thorn, sea berry, and sallow thorn (1). Hippophae L. originated in the Hengduan Mountains and East Himalayas area and is widely distributed in the temperate regions of Eurasia (2). Every part of this plant (fruits, leaves, stems, branches, roots, and thorns) has been traditionally used in medicine, nutritional supplement, soil and moisture conservation, and the establishment of wildlife habitats. Therefore, sea buckthorn is popularly known as “Wonder Plant,” “Golden Bush,” or “Gold Mine” (3).
Since the 1940s, Russian scientists have researched the bioactive substances in the berries, leaves and bark of sea buckthorn, leading to the development of sea buckthorn foods and radiation protection creams for Russian cosmonauts (4). Sea buckthorn contains nearly 200 nutritional and bioactive compounds and is known as a “natural vitamin treasure house” and a “source of nutrition and health care” (5, 6). Sea buckthorn is therefore widely used by the food industry in the preparation of breads, yogurts, jams, beverages, teas and other products (7–9). The medicinal value of sea buckthorn has been recorded in the Tibetan medical classic “Somaratsa,” dating back to as early as the first half of the eighth century (10). Sea buckthorn has been extensively exploited in the folklore treatment of slow digestion, stomach malfunctioning, cardiovascular problems, liver injury, skin diseases, and ulcers (11). In recent years, there have been numerous reports on the pharmacological activities of sea buckthorn, including its anticancer, anti-inflammatory, antimicrobial and antiviral activities, and its ability to act in cardiovascular protection (12–16). There is no doubt that sea buckthorn has great medicinal and therapeutic potential, which may be attributed to the fact that sea buckthorn contains several vitamins, carotenoids, polyphenols, and fatty acids (17–20).
Sea buckthorn is a plant of ecological and economic importance. To promote the role of sea buckthorn in environmental protection, economic development, and human health, the International Sea Buckthorn Association (ISA) was established in 1999 by China, India, Canada, and other countries. In recent years, more countries have become aware of the therapeutic potential of sea buckthorn, and many countries are beginning to recognize and develop a sea buckthorn industry. According to statistics, as of December 2020, sea buckthorn had been distributed to 52 countries around the world, with a total area of 2.33 million hm2. Among this distribution, about 2.1 million hm2 is found within China and the rest is in other countries (21). With the increase of sea buckthorn planting and production, more attention should be paid to the exploitation and utilization of sea buckthorn. Thus, the present review aims to provide a comprehensive overview of the phytochemistry, nutritional and bioactive compounds, health benefits and food applications of sea buckthorn for reference by industrial manufacturers and researchers.
Botanical description
Morphology
Sea buckthorn is a deciduous tree or shrub of the Elaeaceae family and Hippophae L. genus (Figure 1). It is generally 1–8 m high, with some plants growing up to 18 m tall. The leaves are lanceolate or linear, usually 3–8 cm long and less than 7 mm wide. The upper surface of the leaves is dark gray, and the lower surface is distinct silver-gray (22). The fruits are spherical or oblate with a diameter of 5–8 mm. There are usually several fruits stuck together. The fruit is orange-yellow or brownish-red in color and has a ruffled surface. The pulp is oily and soft in texture. The seeds of sea buckthorn are about 4 mm long, 2 mm wide, and obliquely ovate. The seeds are brown and shiny, with a longitudinal groove in the middle. The seed coat is hard, and the seed kernel is creamy white (Figure 1) (23).
Taxonomy
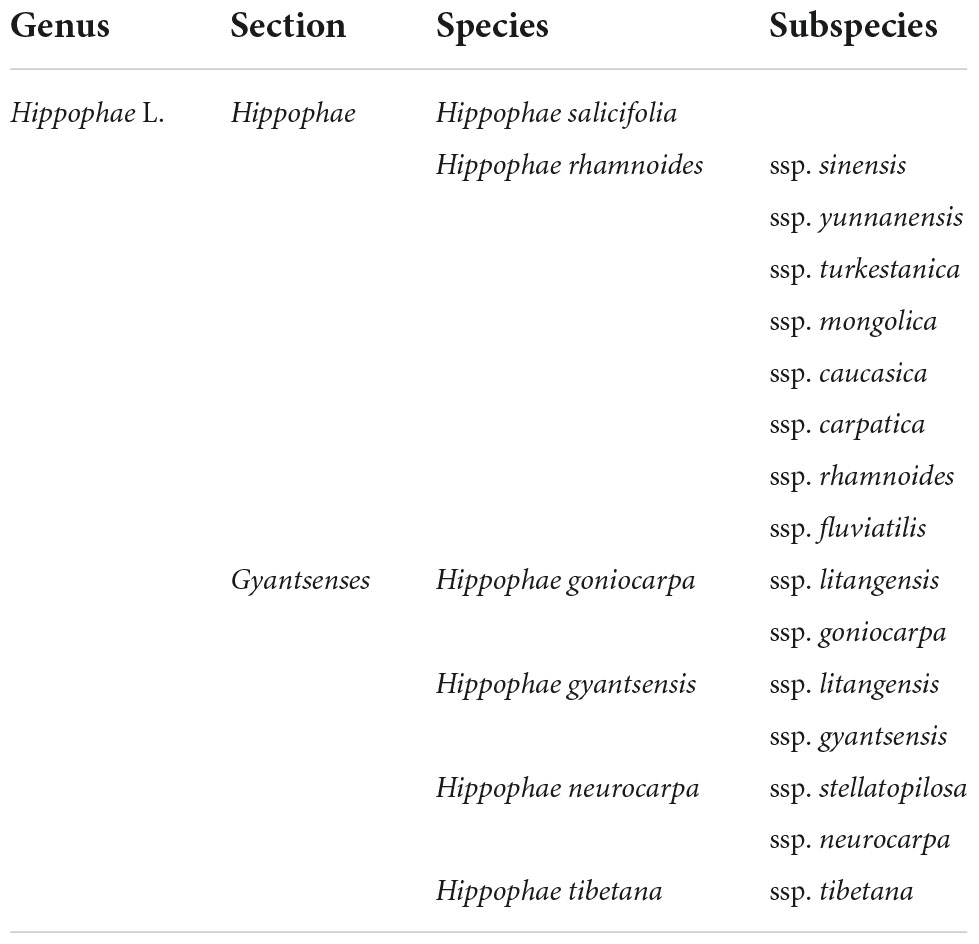
On the basis of the analysis of morphological variation, Arne Rousi classified Hippophae L. (2n = 24) into three species, H. rhamnoides L., H. salicifolia D. Don, and H. tibetana Schlecht. H. rhamnoides is divided into nine subspecies, which include the ssp. carpatica Rousi, ssp. caucasica Rousi, ssp. gyantsensis Rousi, ssp. mongolica Rousi, ssp. sinensis Rousi, ssp. turkestanica Rousi, ssp. yunnanensis Rousi, ssp. rhamnoides, and ssp. fluviatilis van Soest (24). Liu and He described a fourth species, H. neurocarpa. Liu and He, found on the Qinghai-Tibet Plateau of China (25). However, taxonomists still disagree on the precise classification of the genus Hippophae. Chinese scientist Hu has updated and improved the classification system and revised Hippophae L. into 6 species and 17 subspecies (Table 1) (26).
Nutrients and bioactive compounds
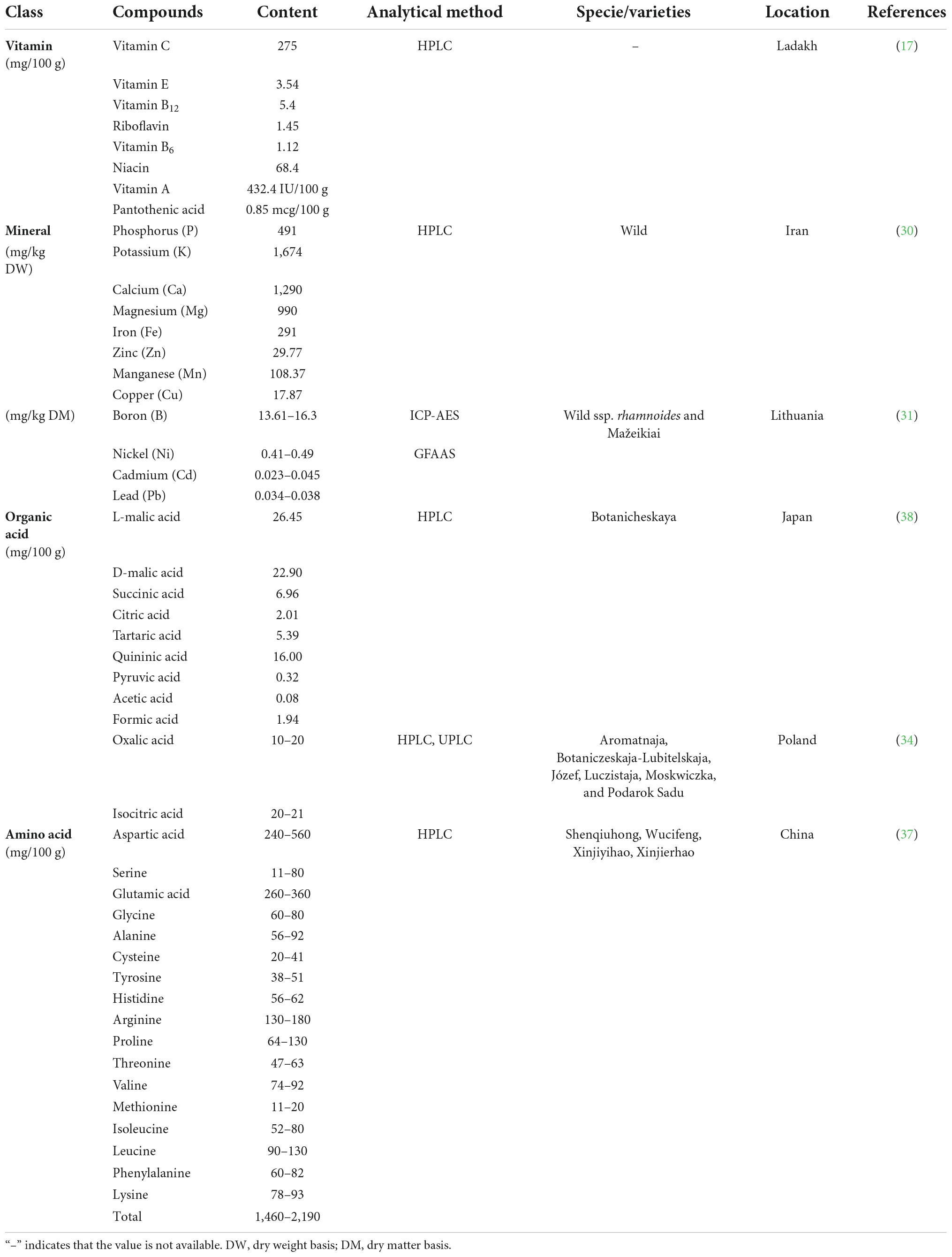
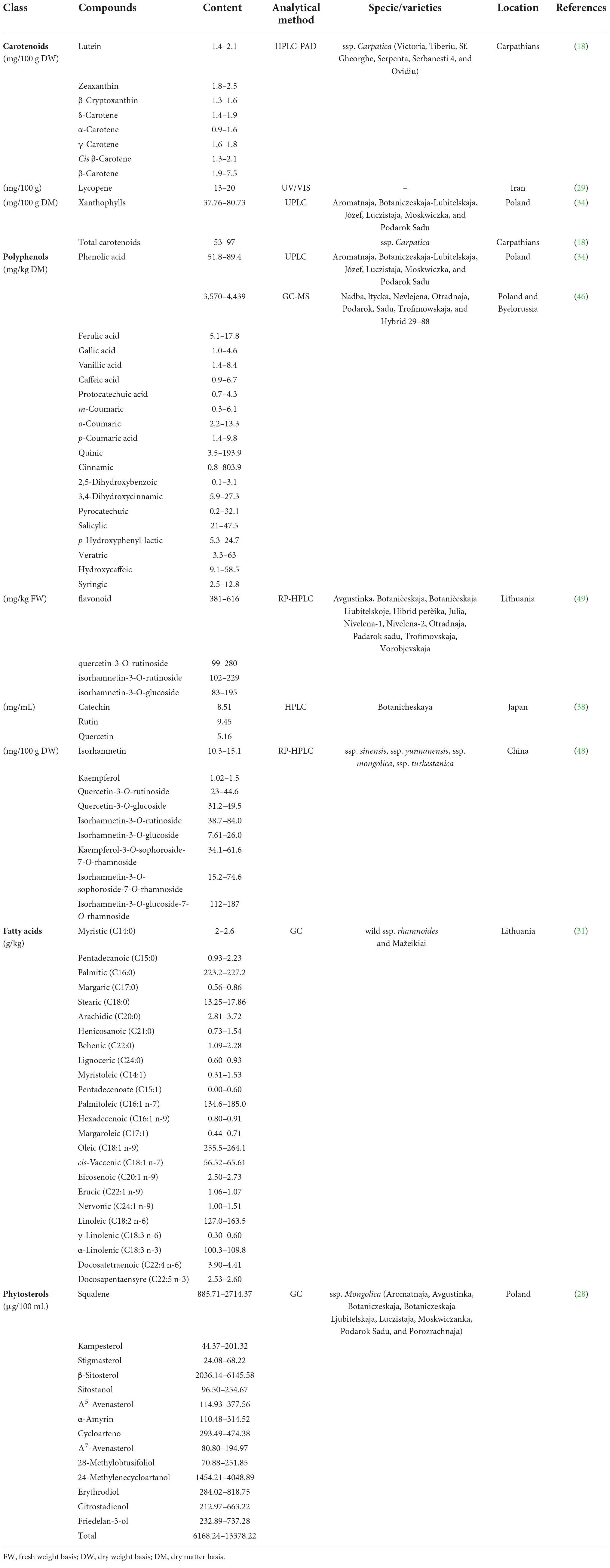
Sea buckthorn contains nearly 200 nutrients and bioactive components (5). Many of the components are well known for their health benefits. Vitamin C is a very important nutrient in sea buckthorn. Carotenoids and polyphenolic compounds, especially phenolic acids and flavonoids, are the main bioactive and antioxidant components of sea buckthorn (27). The fatty acids, phytosterols, organic acids, amino acids, and minerals contained in sea buckthorn also play an important role. The nutrients and bioactive composition content of sea buckthorn influence its health value (28). The nutritional and bioactive composition of sea buckthorn fruit varies considerably depending on genetic variation, the part analyzed, climatic, and growth conditions, year of harvest, degree of maturity, storage conditions, harvest time, and processing and analytic methods (29). Tables 2, 3 show the main nutrients and bioactive components in sea buckthorn fruits, respectively.
Nutrients
Vitamins and minerals
The quality of sea buckthorn fruit is often based on its nutritional value (29). Known as a “natural treasure trove of vitamins,” sea buckthorn is undoubtedly rich in vitamins (6). The vitamin C content of sea buckthorn fruits ranges from 52.86 to 896 mg/100 g (28, 29). It has been showed that the vitamin C content of 100 g of sea buckthorn berries (275 mg) is much higher than the equivalent quantity of mango (27.7 mg), apricot (10 mg), banana (8.7 mg), orange (50 mg), and peach (6.6 mg) (17). In addition, sea buckthorn berries contain vitamin A, vitamin E, riboflavin, niacin, pantothenic acid, vitamin B6, and vitamin B12. Mineral elements are involved in the formation of human tissues and the maintenance of normal physiological functions. Sea buckthorn berries contain many minerals, e.g., phosphorus, iron, magnesium, boron, calcium, aluminum, potassium and others (30, 31). Significant differences in the mineral content of sea buckthorn fruits have been reported at its different stages of maturity. The highest content of calcium, magnesium and phosphorus was found in ripe sea buckthorn fruits with 68.28, 145.67, and 457.7 mg/kg, respectively (32).
Carbohydrates
As the main component of dry matter, carbohydrates play numerous essential roles in living organisms. Monosaccharides are the main source of energy for human metabolism with polysaccharides acting as structural components and the main storage form of energy (33). Sugar content determines the sweetness of the juice. It has been reported that sea buckthorn fruits contain 1.34–2.87 g/100 g FW of sugar. The sugar with the highest content is glucose, accounting for 86.58–92.68% of the total sugar content (34). A study on the sugar composition of three German sea buckthorn varieties reported that the contents of glucose, fructose, and mannitol are 11.95–15.26 mg/mL, 1.75–6.75 mg/mL, and 1.32–6.21 mg/mL, respectively. The sugar content varies among varieties (35).
Organic acids and amino acids
Sea buckthorn fruit contains several organic acids and their derivatives. These organic acid derivatives can promote bone differentiation and contribute to the differentiation of mesenchymal stem cells into osteoblasts (36). Different species of sea buckthorn have different types and concentrations of organic acids. For example, subspecies of Russian sea buckthorn exhibit relatively low total acidity, with organic acid concentrations of 2.1–3.2 g/100 mL. Finnish genotypes were in the middle, ranging from 4.2 to 6.5 g/100 mL, whereas Chinese genotypes showed the highest organic acid concentration, with values between 3.5 and 9.1 g/100 mL (37). It has been reported that sea buckthorn juice contains nine organic acids, namely quinic acid, L-malic acid, D-malic acid, succinic acid, pyruvic acid, tartaric acid, acetic acid, formic acid, and citric acid (38). Another study on six sea buckthorn varieties in Poland detected oxalic acid and isocitric acid (34).
Furthermore, sea buckthorn is rich in amino acids, which are indispensable to the human body. Amino acids are the basic units that make up proteins and are closely related to life activities. Seventeen amino acids, including seven essential amino acids (threonine, valine, methionine, isoleucine, leucine, phenylalanine, and lysine), have been detected in sea buckthorn fruits (39), leaves, branches and seeds (40). The amino acid content in sea buckthorn seeds is 18.63%, in leaves 15.41%, in branches 11.62%, and in fruits 6.89%. The content of aspartic acid and glutamic acid were highest in sea buckthorn fruits, leaves, and branches, with 1.11 and 1.24% in fruits, 2.42 and 1.60% in leaves, and 3.71 and 0.97% in branches. The highest content of tyrosine and glutamic acid can be found in sea buckthorn seeds, at 4.72 and 3.42%, respectively (40).
Bioactive compounds
Carotenoids
Sea buckthorn fruits contain high levels of carotenoids, which give sea buckthorn its characteristic orange-yellow color. Carotenoids mainly act as antioxidants, although they also have other roles. For example, β-carotene is the precursor of vitamin A, and lutein/zeaxanthin constitutes the macular pigment of the eye (41). Carotenoids are considered to have health benefits and can reduce the risk of diseases, especially cancers and eye diseases (42). The content of carotenoids in different species and different parts of sea buckthorn varies greatly. Teleszko et al. (28) detected an average of 11 mg/100 g FW of total carotenoids in eight species of Russian sea buckthorn. In another study on six Romanian sea buckthorn varieties (H. rhamnoides ssp. carpatica), total carotenoid content ranged from 53 to 97 mg/100 g DW in berries, and ranged from 3.5 to 4.2 mg/100 g DW in leaves (18). β-Carotene is the main carotenoid in sea buckthorn. The percentage of β-carotene is 15–55% in berries, and 26–34% in the peel, pulp, and seed oil (28, 43). In addition, carotenoids include γ-carotene, cis-lycopene, lycopene, cis-γ-carotene, β-cryptoxanthin, α-carotene, and so on.
Polyphenols
Polyphenols are the main compounds with antioxidant activity in sea buckthorn. It has been reported that the polyphenol content in the fruit ranges from 12.36 to 34.6 mg GAE/g (GAE, gallic acid equivalents), higher than that in oranges (1.27 mg GAE/g) mandarins (1.16 mg GAE/g), blueberries (2.19 mg GAE/g), sour cherries (2.56 mg GAE/g), and strawberries (1.12 mg GAE/g) (29, 44, 45). A recent review showed that nearly 100 polyphenolic compounds have been isolated and identified from sea buckthorn (27). Polyphenols mainly include phenolic acids and flavonoids. Seventeen phenolic acids have been reported in sea buckthorn berries. Salicylic acid is the main phenolic acid in berries, accounting for 55–74.3% of the total phenolic acids (46). However, another study reported that gallic acid is the main phenolic acid in sea buckthorn fruit and leaves (27).
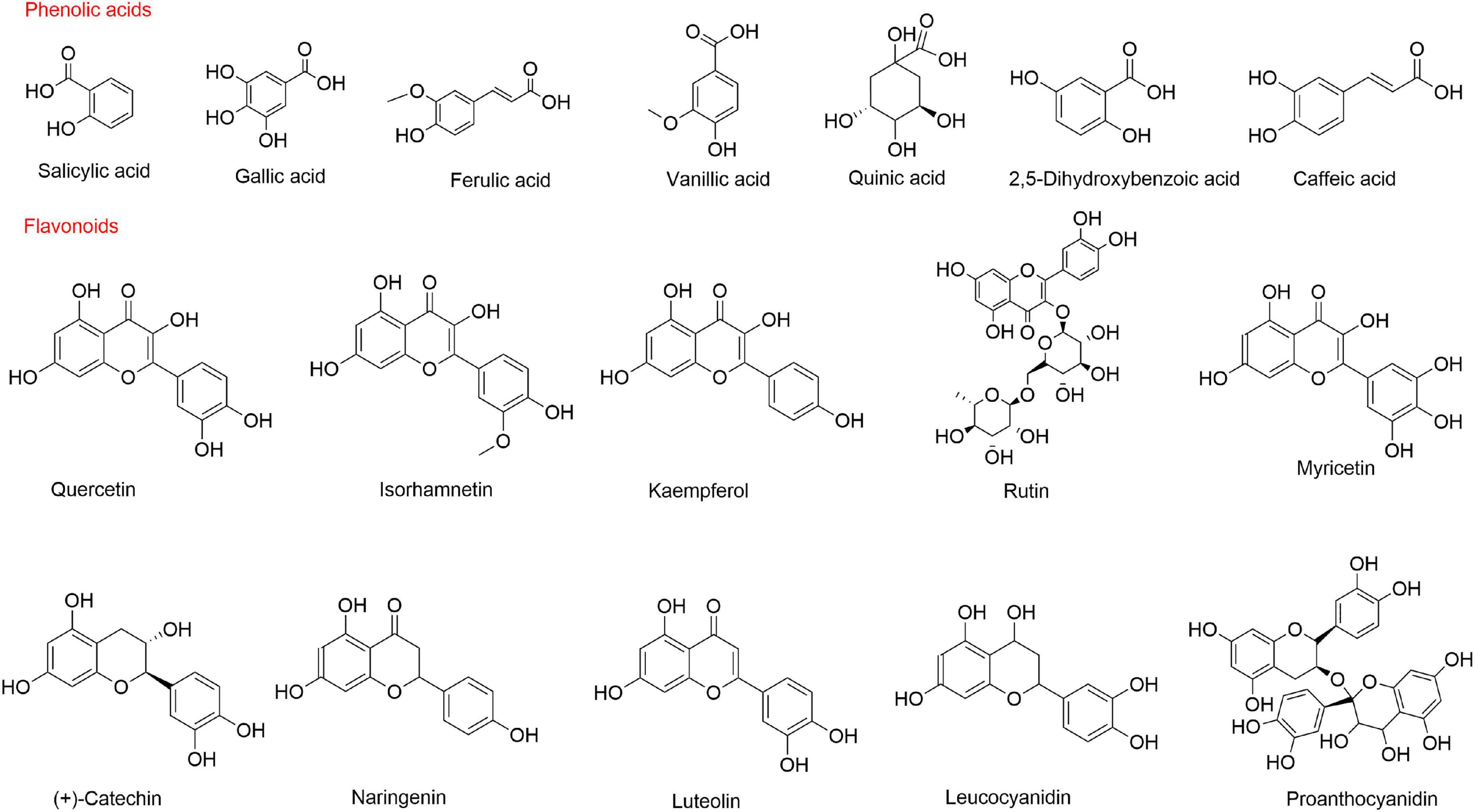
Flavonoids may have potential roles in the prevention of chronic diseases, such as diabetes, cardiovascular disease, and cancer (47). Guo et al. (48) found that the total phenols and flavonoid aglycones in sea buckthorn extract had antioxidant and anti-proliferative activities. To date, 95 flavonoids have been identified from sea buckthorn, including 75 flavonols, 2 dihydroflavones, 6 catechins, 1 leucocyanidin, 9 anthocyanidins, 1 proanthocyanidin, and 1 chalcone (49). Raudonis et al. (50) detected the total flavonoid content in 11 sea buckthorn varieties grown in Lithuania and found that total flavonoid content ranged 385–616 μg/g FW. Flavonols are the major constituents of flavonoids and are mainly present in the glycosylated forms of quercetin, isorhamnetin, and kaempferol (49). Flavonols range from 463.14 mg to 893.92 mg/100 g DM, accounting for approximately 99% of the total phenolic compounds (34). The content and composition of polyphenolic compounds are significantly influenced by geographical factors, climatic conditions and berry varieties. Chemical structures of the main phenolic compounds in sea buckthorn are shown in Figure 2.
Fatty acids
Sea buckthorn is rich in a variety of fatty acids that play an important role in human health, such as treating skin and mucous membrane disorders and dry eyes syndrome and reducing the risk of cardiovascular disease (30). Teleszko et al. (28) identified 11 fatty acids in sea buckthorn pulp oil. At present, 24 fatty acids have been identified in wild and cultivated sea buckthorn berries in Lithuania (Table 3). There were differences in the content of fatty acids between wild and cultivated sea buckthorn. The monounsaturated fatty acid content of wild sea buckthorn berries was significantly higher than that of cultivated berries, while the levels of polyunsaturated and saturated fatty acids were higher in cultivated berries (31). The main fatty acids in sea buckthorn berries are palmitic, palmitoleic, and oleic acids.
Phytosterols
Phytosterols, as a bioactive component, can prevent cardiovascular diseases. A recent study found that the total phytosterol content of berry lipids from eight Russian sea buckthorn species ranged from 6168.24 to 13378.22 μg/100 mL. Fourteen sterol compounds have been detected in sea buckthorn pulp lipids, namely 4-desmethyl sterols (cholestanol derivatives, including β-sitosterol, stigmasterol, campesterol, and Δ5-avenasterol), 4α-monomethyl sterols (e.g., citrostadienol), and 4,4-dimethylsterols (e.g., 24-methylenecycloartanol) (Table 3) (28).
Health benefits
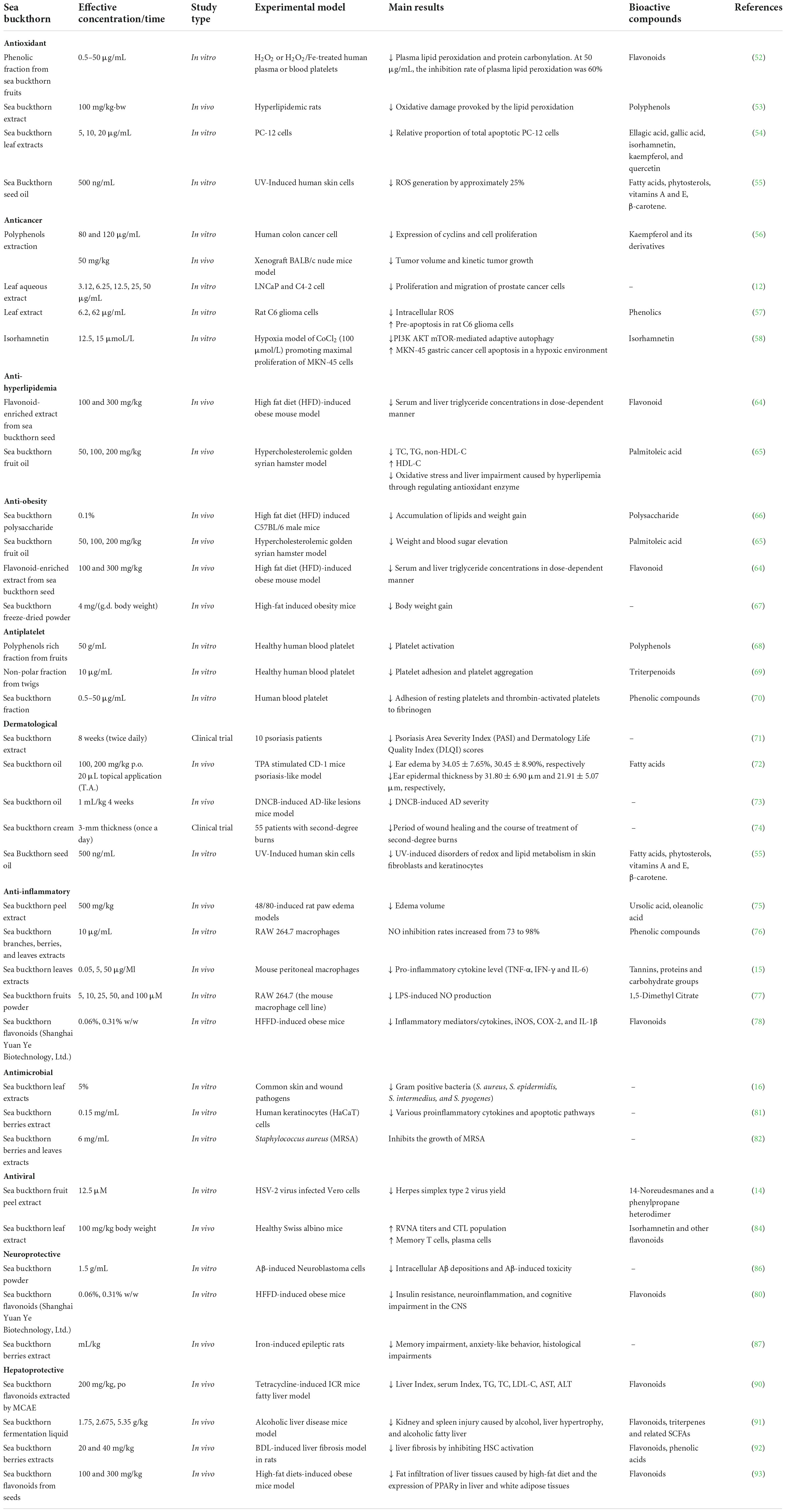
Sea buckthorn contains a variety of bioactive components, including vitamins, carotenoids, polyphenols, fatty acids, and phytosterols. These components exert a wide range of health benefits by exerting antioxidant, anticancer, anti-inflammatory, antimicrobial and antiviral effects, as well as exerting protective cardiovascular, dermatological, neuroprotective, and hepatoprotective effects. The health benefits of sea buckthorn are categorized and summarized in Table 4, which highlights the study type, main results and potential bioactive components.
Antioxidant activity
Many studies have confirmed the antioxidant activity of sea buckthorn in vitro and in vivo. Phenolic fraction from sea buckthorn fruits inhibits hydrogen peroxide (H2O2) or H2O2/Fe stimulated plasma lipid peroxidation and protein carbonylation. In fact, protein carbonylation is a relatively stable biomarker of oxidative stress. The phenolic constituents of sea buckthorn fruit reduced the concentration of carbonyl groups in plasma protein treated with H2O2 or H2O2/Fe. When plasma was treated with sea buckthorn phenolic fractions at a concentration of 50 g/mL for 60 min, the inhibition rate of plasma lipid peroxidation was as high as 60% (51). In vitro trials have shown that sea buckthorn extract with or without atorvastatin for the treatment of hyperlipidemia helped reduce the oxidative damage caused by lipid peroxidation (52). In addition, sea buckthorn leaf extract attenuates intracellular oxidative stress in a dose-dependent manner, thereby increasing neuronal PC-12 cell viability and membrane integrity (53). Serban et al. (54) retrieved 3,145 results from six databases, including PubMed, Scopus, Web of Science and others, among which 101 studies on cardiovascular disease showed that sea buckthorn fruit lowered blood cholesterol levels and reduced inflammation and oxidative stress parameters. Sea buckthorn seed oil inhibits ultraviolet (UV)-induced redox balance disturbance in skin cells. Gęgotek et al. (55) reported that fibroblast incubation with sea buckthorn oil causes decreases in reactive oxygen species (ROS) generation by approximately 25%. Sea buckthorn may be used as a natural source of antioxidants to prevent and treat diseases related to oxidative stress.
Anticancer activity
At present, many studies have shown that the bioactive components in sea buckthorn have anticancer activity. Sea buckthorn polyphenols, the active ingredient of kaempferol and its derivatives, have shown significant anti-colon cancer activity in vitro and in vivo. Sea buckthorn polyphenols upregulate expression of microRNA (miR)-195-5p and miR-497-5p and down-regulate the expression of miR-1247-3p to suppress cyclins expression, thereby arresting the cell cycle in the G1 phase and affecting further proliferation of colon cancer. In addition, sea buckthorn polyphenols (50 mg/kg) significantly reduced tumor volume and control tumor growth in xenografted BALB/c nude mice in vivo (56). Sea buckthorn leaf aqueous extract effectively targeted androgen receptor (AR) and significantly downregulated androgen response genes, prostate specific antigen (PSA), eleven-nineteen lysine-rich leukemia 2 (ELL2), ELL-associated factor 2 (EAF2), calreticulin (CALR) in vitro. Sea buckthorn leaf aqueous extract can effectively inhibit proliferation and migration of prostate cancer cells. Therefore, sea buckthorn leaves hold promise as a functional food that may play a key role in the prevention of prostate cancer in high-risk populations. However, the potential bioactive compounds in sea buckthorn leaves are yet to be investigated for the development of new treatment options for prostate cancer (12).
Kim et al. (57) reported that sea buckthorn leaf extract at concentrations of 6.2 and 62 μg/mL significantly reduced the production of intracellular ROS by 16.3 and 42.3%, respectively, up-regulated expression of the pro-apoptotic protein B-cell lymphoma-2 (BCL2)-associated X (Bax) and inhibited the rapid proliferation of C6 glioma cells (11 and 49.5%). Therefore, sea buckthorn may be a potential source of pharmacological interventions for glioma treatment. In addition, isorhamnetin, the active component of sea buckthorn, increased expression of the mitochondrial pathway pro-apoptotic protein (cytochrome c-caspase 9-caspase 3) in gastric cancer cells in a hypoxic environment. It also significantly inhibited the autophagy of MKN-45 gastric cancer cells and promoted the apoptosis of gastric cancer cells by activating the Phosphoinositide 3-kinase (PI3K)-protein kinase B (AKT)-mammalian target of rapamycin (mTOR) signaling pathway (58).
In short, these studies support the anticancer effect of sea buckthorn and suggest that polyphenolic compounds may be responsible for its anticancer activity. The anticancer mechanisms of sea buckthorn are related to the expression of cyclin, proapoptotic proteins, autophagy of cancer cells, and related signaling pathways. However, there are few in vivo experiments and clinical trials on the anticancer effects of sea buckthorn. Thus, further research on the anticancer effects of sea buckthorn in humans is needed. A growing number of studies have found that carotenoids, especially lycopene, can reduce the risk of prostate, breast, lung, cervical and other cancers (59). However, there are almost no studies on the anticancer activity of sea buckthorn carotenoids. The anticancer activity of sea buckthorn carotenoid extracts is a promising research direction.
Anti-hyperlipidemia activity
Hypercholesterolemia is an important risk factor for cardiovascular disease (60). The bioactive substance in the lipids of sea buckthorn pulp, phytosterols, plays an important role in the prevention of cardiovascular diseases, especially hypercholesterolemia (28). Numerous clinical trials have shown that spreads with added phytosterols have a stronger cholesterol-lowering effect, reducing low-density lipoprotein cholesterol (LDL-C) levels by about 10–15% (61). The mechanism of the hypocholesterolemic effect of phytosterols may be via the inhibition of endogenous cholesterol reabsorption and the promotion of its excretion in the form of neutral steroids (62). A meta-analysis from 11 independent randomized controlled trials concluded that supplementation with sea buckthorn berries/extracts significantly improved total cholesterol, triglyceride (TG), LDL-C, and high-density lipoprotein cholesterol (HDL-C) in subjects with hyperlipidemia, but not in healthy subjects (63).
In vivo animal trials showed that sea buckthorn has anti-hyperlipidemic effects. Flavonoid-enriched extract from sea buckthorn seed (FSH) at a dose of 100 and 300 mg/kg reduced serum and liver triglyceride concentrations by 16.67 and 49.56% in high fat diet (HFD)-induced obese mouse, respectively. FSH may improve lipid metabolism by inhibiting peroxisome proliferator-activated receptor gamma (PPARγ) expression, promoting PPARα expression, and suppressing adipose tissue inflammation (64). In addition, sea buckthorn fruit oil extract dose-dependently attenuated metabolic dysfunction in hamsters with hyperlipemia, including improving blood lipid composition (total cholesterol (TC), TG, HDL-C, and non-HDL-C levels), and relieving oxidative stress and liver impairment through the AMP-activated protein kinase (AMPK) and Akt pathways (65). In summary, sea buckthorn fruit, seed and oil are a source of phenolic compounds (especially flavonoids) and phytosterols. Sea buckthorn may be a valuable source of important bioactive compounds for the prevention and treatment of cardiovascular disease, which requires further research support.
Anti-obesity activity
Sea buckthorn polysaccharide promotes expression of PPARγ-coactivator 1α (PGC1α), uncoupling protein-1 (UCP-1), and PR domain containing 16 (PRDM 16) in adipocytes to activate the brown adipocytes and improve thermogenesis, thus inhibiting the accumulation of lipids and weight gain (66). Palmitic acid-rich sea buckthorn fruit oil extract reduces the weight of hypercholesterolemic hamsters and blood sugar elevation caused by dyslipidemia. Therefore, sea buckthorn fruit oil can relieve obesity caused by hyperlipidemia (65). It has been reported that FSH at doses of 100 and 300 mg/kg significantly reduced body weight gain in HFD-induced obese mice by 33.06 and 43.51%, respectively (64). Sea buckthorn freeze-dried powder is made by low-temperature freeze-drying technology, allowing the powder to retain all of the plant’s useful nutrients and functional ingredients. Sea buckthorn powder improves HFD-induced obesity by altering the composition and structure of gut microbiome (67). Sea buckthorn is likely to develop into functional foods and dietary supplements for obese people.
Antiplatelet activity
Anticoagulant and antiplatelet agents play an important role in the prevention and treatment of cardiovascular thrombotic events caused by various mechanisms. The polyphenols rich fraction of sea buckthorn fruit at the highest used concentration (50 μg/mL) has potent antiplatelet activity compared to polyphenol and triterpenic acid rich fractions from leaves and twigs. It has been shown to inhibit the expression of PAC-1 in three models of non-activated platelets, platelets activated by 10 μM adenosine diphosphate (ADP), and platelets activated by 10 μg/mL of collagen. This may be due to the inhibition of platelet aggregation as a result of the low expression of GPIIb/IIIa (68). Another report indicated that the non-polar fraction of sea buckthorn twigs showed stronger antiplatelet activity than the phenolic and non-polar fractions of leaves. This activity may be related to the regulation of arachidonic acid metabolism, changes in ROS concentration, and expression of platelet receptors (69). The 50 g/mL sea buckthorn fraction inhibited the adhesion of resting platelets and thrombin-activated platelets to fibrinogen by 65 and 55%, respectively (70).
Dermatological effect
Sea buckthorn has been reported to have a wide range of dermatological effects. Clinical trials have demonstrated the anti-psoriasis effects of sea buckthorn. Boca et al. (71) treated 10 patients diagnosed with mild to moderate psoriasis with topical sea buckthorn fruit extract. When compared with placebo-treated patients, the Psoriasis Area Severity Index (PASI) score and Dermatology Life Quality Index (DLQI) scores in the treatment group were improved at both the fourth and eighth weeks of treatment. Sea buckthorn also exhibits anti-psoriatic and anti-atopic dermatitis activities in animal models. In the 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced psoriasis-like lesion CD-1 mouse model, simultaneous oral (100 and 200 mg/kg) and topical (20 μL) application of sea buckthorn oil significantly inhibited ear edema (34.05 ± 7.65%, and 30.45 ± 8.90%, respectively) and reduced ear biopsy weights. Sea buckthorn oil has anti-inflammatory and anti-psoriatic properties. The possible mechanism for these effects may be that the high levels of fatty acids in sea buckthorn oil acts to inhibit reactive nitrogen and down-regulate nuclear factor kappa-B (NF-κB) protein and pro-inflammatory cytokines (72). Studies have suggested that 4 weeks of consecutive use of sea buckthorn oil decreases 2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis (AD) severity in mice. This effect was due to inhibition of the thymus activation regulated chemokine (TARC) and macrophage-derived chemokine (MDC) in Interferon-γ (IFN-γ)/tumor necrosis factor-α (TNF-α)-stimulated HaCaT cells, which occurred by blocking activation of the NF-κB/signal transducerand activator of transcription 1 (STAT1) signaling pathway, thereby inhibiting the development of AD-like skin lesions. Sea buckthorn oil may be an effective therapeutic agent in the treatment of patients with AD (73).
Moreover, a randomized triple-blind clinical trial demonstrated that the healing period for second-degree burns in patients treated with 40% sea buckthorn cream was about 5 days shorter than for patients treated with 1% silver sulfadiazine dressings. The sea buckthorn cream had better clinical efficacy and shortened the healing time for second-degree burns (74). It was found that sea buckthorn seed oil promoted wound contraction by increasing hydroxyproline, hexosamine, DNA, and total protein content, which in turn promoted full-layer burn wound healing. The wound healing potential of sea buckthorn seed oil is dependent on the presence of omega-3 and omega-6 fatty acids, tocopherols and carotenoids (75). In addition, the palmitic acid-rich fraction purified from sea buckthorn seed oil has cell proliferation properties that promote growth of keratinocytes and dermal fibroblasts, which can be used to develop skin preparations and skin care products (76). UV light induces damage to the redox system and impairs lipid metabolism in skin fibroblasts and keratin-forming cells, and sea buckthorn oil can inhibit this effect and sea buckthorn seed oil may be a promising natural substance for skin photoprotection (55).
Overall, sea buckthorn has a therapeutic role in dermatology due to the high levels of saturated, monounsaturated and polyunsaturated fatty acids and other biological compounds that exert their effects. Determining the specific bioactive compounds and their mechanisms of action, however, requires further research.
Anti-inflammatory activity
Sea buckthorn is widely used in traditional medicine to treat inflammatory diseases. The anti-inflammatory activity of sea buckthorn has been demonstrated in many in vivo studies. For example, 70% methanolic extract of sea buckthorn (500 mg/kg) inhibited 48/80-induced edematous inflammation and significantly reduced the volume of foot swelling in rats (0.660 ± 0.082 mL, compared to 0.935 ± 0.041 mL in the control). The anti-inflammatory effect of the peel extract was greatest (0.470 ± 0.124 mL, compared with 0.920 ± 0.111 mL for the control). Ursolic acid and oleanolic acid were the main active compounds in the peel extract. They may cause membrane stabilization by inhibiting mast cell degranulation (77). The anti-inflammatory activity of sea buckthorn branches, leaves, and fruits was measured by nitric oxide (NO) production, and it was found that treatment with 10 μg/mL sea buckthorn extracts inhibited NO by 73–98%. Cytotoxic effects of sea buckthorn have not been observed in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays. Sea buckthorn extracts displayed good anti-inflammatory activities in RAW 264.7 macrophages (78). Sea buckthorn leaves extract exhibited potent anti-inflammatory activity against lipopolysaccharide (LPS) stimuli by inhibiting the expression of NO, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), and by decreasing levels of pro-inflammatory cytokines (15). Furthermore, sea buckthorn fruit extract, identified as a citric acid derivative, inhibited LPS-induced NO production in RAW 264.7 cells by inhibiting the expression of IκB kinase alpha/beta (IKKα/β), inhibitor of kappa Bα (I-κBα), NF-κB p65, iNOS, and COX-2, and the activities of interleukin 6 (IL-6) and TNF-α (79). Similarly, Mulati et al. (80) reported that sea buckthorn flavonoids significantly reversed high-fat and high-fructose diet (HFFD)-induced iNOS overexpression and reduced interleukin 1β (IL-1β) and COX-2 mRNA levels in the hippocampus of mice, suppressing the HFFD-induced inflammation reaction.
Therefore, the anti-inflammatory activity of sea buckthorn may be attributed to ursolic acid, oleanolic acid, citric acid derivatives and flavonoids. Its anti-inflammatory mechanism of action may be related to inhibition of the expression of pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) and a reduction in the production of pro-inflammatory mediators (NF-κB, iNOS, and COX-2). Sea buckthorn has shown promise as a source of bioactive compounds for the treatment of inflammatory diseases, but more in vivo and clinical studies are still needed to support this.
Antimicrobial and antiviral activity
It has been reported that sea buckthorn exhibits antimicrobial activities in vitro. Verma et al. (16) found that sea buckthorn leaf extract was significantly effective against all 67 gram-positive bacteria recovered from clinical samples. Sea buckthorn leaf extract at a 5% concentration inhibited S. aureus, S. epidermidis, S. intermedius, and S. pyogenes growth by almost 50%. Sea buckthorn extract may reverse the detrimental effects of S. aureus on human keratinocytes by down-regulating various pro-inflammatory cytokines and apoptotic pathways, such as ILs, TNFs, transforming growth factors (TGFs), IFNs, fibroblast growth factors (FGFs), MAPKs, matrix metalloproteinases (MMPs), and caspases and Wnts molecular pathways (81). Additionally, a study showed that 6 mg/mL of sea buckthorn berry and leaf extract significantly inhibits the growth of Methicillin-resistant S. aureus (MRSA) (82). Smida et al. (83) reported that an experimentally designed mouthwash based on sea buckthorn pulp oil had bactericidal effects on some periodontal pathogens and had the ability to inhibit the formation of single-strain and multi-strain biofilms.
Moreover, sea buckthorn exhibits significant antiviral activity. 14-Noreudesmanes and a phenylpropane heterodimer isolated from the 70% methanol extract of sea buckthorn fruit inhibited the replication of herpes simplex type 2 (HSV-2) virus. Therefore, sea buckthorn may be a potential source of antiviral agents with anti-HSV-2 activity and may provide an alternative drug candidate for the treatment of patient populations infected with acyclovir- and penciclovir-resistant strains of HSV-2 (14). In addition, it has been shown that immunization with sea buckthorn leaf extract and inactivated rabies virus antigens (SBTE + Rb) increases rabies virus neutralizing antibody (RVNA) titers and the cytotoxic T lymphocytes (CTLs) response. Compared with the Rb immunized group, memory T cells and plasma cells in the SBTE + Rb immunized group were significantly increased by 5.5 and 1.9%, respectively. The components of sea buckthorn leaf extract that exert adjuvant activity may be isorhamnetin and other flavonoids (84).
Neuroprotective activity
Alzheimer’s disease is a neurodegenerative disorder in which the typical histopathological changes are extracellular amyloid-β (Aβ) deposition and neurofibrillary tangles due to Tau protein hyperphosphorylation (85). Sea buckthorn removes intracellular Aβ deposits, with sea buckthorn powder at 1.5 g/mL being the most effective. This finding may be attributed to the higher levels of antioxidants present in sea buckthorn berry powder. Antioxidants inhibit Aβ-induced toxicity and prevent cell death by exerting a neuroprotective effect. Sea buckthorn holds promise as a potential therapeutic agent for the treatment of Alzheimer’s disease (86). Another study showed that sea buckthorn flavonoids stimulated insulin receptor substrate (IRS)/AKT activation, reduced protein tyrosine phosphatase 1B (PTP1B) expression and normalized insulin signaling pathways, neurogenic damage and ERK/CREB/BDNF signaling pathways. It inhibited insulin resistance and neuroinflammation, attenuated HFD-induced cognitive impairment, and effectively prevented memory loss (80). In addition, sea buckthorn improved epileptiform activity in the cerebral cortex and hippocampus in iron-induced epilepsy rats and reduced anxiety-like behavior, and improved memory impairment and histological damage in rats (87). In conclusion, sea buckthorn has been demonstrated to possess neuroprotective effects. The mechanisms include the removal of Aβ deposits, inhibition of Aβ-induced toxicity, and inhibition of insulin resistance and neuroinflammation. These effects may be attributed to the presence of flavonoids and other antioxidant compounds in sea buckthorn. In the future, it is necessary to investigate the neuroprotective effect of sea buckthorn on humans using clinical trials.
Hepatoprotective activity
Sea buckthorn extract and sea buckthorn oil have significant hepatoprotective activities. Sea buckthorn oil is rich in carotenoids and may be an important source of bioavailable lutein (88). Carotenoids such as β-carotene, lycopene, lutein, and β-cryptoxanthin exhibit hepatoprotective activity by reducing oxidative stress and regulating lipid metabolism in hepatocytes (89). Biochemical and histopathological studies have shown that sea buckthorn flavonoid extract significantly improves biomarkers, including TG, TC, LDL-C, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) in the serum and liver of non-alcoholic fatty liver mice. The therapeutic effects of sea buckthorn were superior to that of curcumin (90). Furthermore, sea buckthorn fruit fermentation solution regulates hepatic lipid metabolism and oxidative stress by modulating the composition of the intestinal microbiota. Thus, sea buckthorn prevents alcoholic liver disease and exerts hepatoprotective effect (91). Another study found that the active ingredients in sea buckthorn inhibited the activation of hepatic stellate cells, reduced inflammatory cytokine levels, and reduced the development of bile duct ligation (BDL)-induced fibrosis in rats in a dose-dependent manner. Thus, sea buckthorn reduces liver injury and inflammation, and restored liver function (92). It has been reported that a flavonoid extract from sea buckthorn seed residues reduces the number of adipocytes in the liver of obese mice, which significantly reduces HFD-induced fatty infiltration in the liver tissue, and reduces expression of PPARγ in the liver and adipose tissue, resulting in reduced of fat accumulation (93). In general, the flavonoids and carotenoids in sea buckthorn have hepatoprotective effects. The mechanisms for these effects may be associated with the regulation of lipid metabolism and oxidative stress and a reduction in inflammatory factor levels. It is necessary to conduct more in vivo studies to explore the hepatoprotective activity of sea buckthorn.
Food applications
In addition to medical biological activities, sea buckthorn is also widely used in food, and has high economic value. Sea buckthorn is rich in nutritional value and contains a variety of biologically active compounds. Sea buckthorn is currently used as an antioxidant, antimicrobial and other natural additives in a variety of food products. The application of sea buckthorn in the food industry is more and more extensive, such as sea buckthorn oil, freeze-dried powder, fruit juice, fruit wine, milk tablets, fruit vinegar drinks, tea (94), preserved fruit, yogurt, and jam. The maximum utilization of sea buckthorn to improve the sensory properties and nutritional value of sea buckthorn products is currently being pursued by food industry manufacturers and researchers.
Food additives
The meat processing industry is currently seeking natural additives to replace chemical additives in their products. Kozhakhiyeva et al. (95) found that the new functional cooked and smoked horse meat Jaya product, produced by adding 5.0% sea buckthorn fruit powder extract, is rich in 1.0% bioactive substances. The samples showed a 38% reduction in lipolysis and a significant 24% reduction in lipid hydroperoxides after 21 days of storage. This improved the oxidative stability and quality of new functional horsemeat delicacies. The addition of 3% ethanolic extract of sea buckthorn fruit to pork sausage effectively inhibited lipid oxidation and reduced the total bacterial count. Total sausage colonies were reduced by approximately 7 times, improving the microbiological content of the sausage (96).
The addition of sea buckthorn fruit powder to wheat bread extends the shelf life of the bread by 1–3 days. It also improves the antioxidant and organoleptic properties of the bread (8). The addition of 0.8 g/L of sea buckthorn leaf powder to white wine increased its free radical scavenging activity from 28.4 to 55.8%. The reducing ability of white wine, as measured by the amount of reduced ferric ion in an antioxidant power assay, increased from 35.3 to 62.1% with the addition of sea buckthorn. The total phenolic content of white wine increased from 11 to 23.7% and the color intensity increased from 39.9 to 50.7%, which contributed to the antioxidant capacity of the wines without sulfites (97). Sea buckthorn leaves have significant antioxidant capacity (98), and can be used as an alternative to increase the antioxidant capacity of wines.
Studies have found that sea buckthorn juice and its by-products could be used in chewing gum formulations and significantly improve the antioxidant activity. It showed antimicrobial properties against MRSA, Klebsiella pneumoniae, Salmonella enterica, Pseudomonas aeruginosa, Bacillus cereus, etc. Sea buckthorn juice and its by-products have great potential as antimicrobial agents in the food industry (99).
Furthermore, sea buckthorn seeds used to purify chitinase, via its action on the antifreeze protein HrCHI4 preserved the integrity of frozen green pea membranes and helped preserve sample freshness by retaining volatile compounds. This study opens up the possibility of using edible products to preserve food and preserve its texture and freshness by natural means (100).
All in all, sea buckthorn has a promising future as a natural food additive. The bioactive compounds contained in sea buckthorn, such as polyphenols (especially flavonoids), ascorbic acid, vitamins, carotenoids, and antifreeze proteins exert antioxidant, antibacterial and antifreeze effects. In the future, it will be necessary to investigate these mechanisms of action in depth for better application in food production.
Sea buckthorn yogurt
Sea buckthorn, as a new plant-based additive, is becoming increasingly popular in dairy production worldwide due to its healthful, nutritional benefits. This is exactly the kind of nutritional quality that consumers are happy to seek. Sea buckthorn is rich in nutritional active substances and its addition to yogurt enhances the nutritional value of yogurt. Developed from sea buckthorn berries, sea buckthorn yogurt is rich in fat, protein, carbohydrates and antioxidants (vitamin C, vitamin E, carotenoids, phenols, etc.) meeting people’s nutritional needs. The yogurt can be stored safely at 4°C for 12 days and at 15°C for 3 days without losing its microbiological quality (9). In addition to adding sea buckthorn to yogurt, carrot (101), tomato (102), water chestnut (103), yellow peach, and passion fruit have also been added to sea buckthorn yogurt to develop novel healthy yogurt. The different additions add to the unique natural flavor of fruits and vegetables, enriching the yogurt with a variety of functional ingredients and making up for the nutritional deficiencies of plain yogurt (101).
Sea buckthorn jam and jelly
Sea buckthorn berries have a sour taste and a short shelf life. Therefore, processing berries into jam is an effective means to improve sensory characteristics and increase berry utilization. The jam, produced using sea buckthorn fruit at 102°C with stevia, contains high levels of total carotenoids and polyphenols and exhibits antioxidant activity. After 21 days of storage at room temperature, the value of yeast and mold was less than 100 CFU/g, and the value of Enterobacteriaceae was less than 5 CFU/g (104). Ordinary jam has a single flavor. In the Elaeagnus angustifolia and sea buckthorn compound jam, sea buckthorn was used both as a raw material and as an acidulant instead of citric acid. The jam has a shelf life of 177 days at 20°C without the addition of preservatives (105). In addition, sea buckthorn can be combined with sweet potatoes, pumpkins and carrots in a certain ratio to make novel, nutritious and healthy compound jam (106). A reasonable mixture of sea buckthorn juice with other fruit juices (papaya, watermelon, grape) can produce a delicious and nutritious jelly. Among them, sea buckthorn mixed jelly prepared in certain ratios with grapes has shown good organoleptic characteristics. The shelf life of sea buckthorn-grape jelly is 6 months at room temperature and its microbial load is also within the specified limits (107). Sea buckthorn has the potential to be a potentially rich source of bioactive compounds for the production of sugar-based products.
Sea buckthorn beverages
Sea buckthorn berry wastes are inevitably generated in the sea buckthorn processing industry, and improper disposal of these wastes will cause environmental pollution. Fermentation and reuse of these wastes can improve the utilization rate of sea buckthorn and increase the economic value of these wastes. Waste from the sea buckthorn processing industry can be used as a suitable substrate for fermentation. Fermentation under optimal fermentation conditions resulted in 3% ethanolic sea buckthorn beverage. This beverage contains high levels of phenolic compounds (including gallic acid, protocatechuic acid, vanillic acid, chlorogenic acid, etc.) and high antioxidant activity, and contains carbon dioxide and low levels of ethanol. So it is a refreshing and healthy functional drink (108). In addition to fermentation, a waste-free whole fruit pulp juice of sea buckthorn can be developed using the micro-wet milling (MWM) process. Compared to mixed milled and commercial sea buckthorn juice, MWM sea buckthorn juice has a better color (bright yellow tint, highest total carotenoid content of 145 ± 0.10 mg/mL), smaller particle size, higher ascorbic acid value (67.67 ± 1.15 mg/mL), total phenolic content and antioxidant activity. The process minimizes the loss of heat-sensitive bioactive compounds. It also provides a fiber-rich juice, showing great promise for processing sea buckthorn juice in the food industry (109). Sea buckthorn is rich in nutrients and bioactive compounds. The potential of sea buckthorn as a botanical ingredient for novel functional food applications is obvious. It is promising to make full use of sea buckthorn fruits, peels, and seeds and to explore new ways of processing sea buckthorn.
Toxicity and safety
Despite the long nutritional and medicinal history of sea buckthorn, there is still relatively little information available on the toxicity and safety of sea buckthorn. Wen et al. (110) reported that sea buckthorn berry oil was not a genotoxic or teratogenic substance. He found that sea buckthorn berry oil showed no mutagenic activity against histidine-dependent strains of Salmonella typhimurium at exposure concentrations ranging from 8 to 5,000 μg/plate. At a dose up to 9.36 g/kg body weight, sea buckthorn berry oil had no significant effect on sperm morphology and the micronucleus rate of polychromatic erythrocytes in mice. In addition, 4.68 g/kg of sea buckthorn fruit oil did not cause maternal toxicity or embryotoxicity in pregnant mice. The no-observed-adverse-effect level (NOAEL) for rats was determined to be 4.68 g/kg of body weight. A 90-day safety study showed that the NOAEL in rats was 100 mg/kg body weight/day of aqueous fruit extract of sea buckthorn (111). Furthermore, Zhao et al. (112) reported that the maximum tolerated dose of sea buckthorn oil in the acute toxicity study in mice was bigger than 18.72 g/kg. Ninety-day repeated oral toxicity tests in rats showed that the NOAEL was 9.36 g/kg body weight. Sea buckthorn is healthy and non-toxic enough to be used as a human food, medicinal product, or dietary supplement.
Conclusion and outlooks
Sea buckthorn is a unique and highly valuable plant with a wide distribution covering more than 2 million hectares of land worldwide. It contains nearly 200 bioactive compounds and has great benefits to human health, and in vivo, in vitro, and clinical trials in the past 5 years have demonstrated the health benefits of sea buckthorn. These biological activities include antioxidant, anticancer, anti-inflammatory, anti-hyperlipidemic, anti-obesity, antimicrobial and antiviral, dermatological, neuroprotective, and hepatoprotective effects. Moreover, sea buckthorn has created enormous economic value in the food industry and is a promising dietary source of bioactive components.
As a versatile economic and ecological plant, sea buckthorn undoubtedly has a bright future. This ancient plant has powerful therapeutic synergies and has made many contributions to humankind. Sea buckthorn has an outstanding ability to help with economic development and improve the ecological environment. In order to rationally develop and utilize sea buckthorn resources, further research should focus on: (1) Developing mechanical harvesting and reasonable preservation technology. Sea buckthorn is a berry plant and manual harvesting is inefficient. The berries have high moisture content and are easily squeezed leading to deformation and mold. It is necessary to improve the efficiency at the harvesting stage; (2) Isolation and identification of more specific bioactive compounds and further study of their health promoting mechanisms; (3) Conducting more clinical trials to verify the health benefits of sea buckthorn for humans; (4) Applying sea buckthorn in the prevention and treatment of diseases; (5) Developing functional foods and other products based on sea buckthorn; (6) Adhering to the mode of combining economy and ecology, and developing the sea buckthorn industry in a scientific, reasonable and sustainable manner.
Author contributions
QM conceptualized the topic. FZ and PW reviewed the literature and collected the data. ZW drafted the manuscript. GH and XC revised and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the National Natural Science Foundation of China (grant no. 81473104).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Singh B, Peter K. Indian sea buckthorn. New age herbals. Singapore: Springer (2018). p. 29–54. doi: 10.1007/978-981-10-8291-7_3
2. Chen X, Lian Y. The geographical distribution patterns and its formative factors on the genus Hippophae L. Acta Bot Boreali Occident Sin. (1994) 14:105–10.
3. Stobdan T, Targais K, Lamo D, Srivastava R. Judicious use of natural resources: a case study of traditional uses of seabuckthorn (Hippophae rhamnoides L.) in trans-himalayan ladakh. India Natl Acad Sci Lett. (2013) 36:609–13. doi: 10.1007/s40009-013-0177-4
4. Piłat B, Bieniek A, Zadernowski R. Common sea buckthorn (Hippophae rhamnoides L.) as an alternative orchard plant. Pol J Nat Sci. (2015) 30:417–30.
5. Wang K, Xu Z, Liao X. Bioactive compounds, health benefits and functional food products of sea buckthorn: a review. Crit Rev Food Sci Nutr. (2021) 2021:1–22. doi: 10.1080/10408398.2021.1905605
6. Wang X, Xu T, Liu Y, Liu Y, Liu J, Li M, et al. Research progress on the relative theory of medicinal plants of Hippophae L World. Chin Med. (2021) 16:2217–27. doi: 10.3969/j.issn.1673-7202.2021.15.002
7. Gâtlan A, Gutt G. Sea buckthorn in plant based diets. an analytical approach of sea buckthorn fruits composition: nutritional value, applications, and health benefits. Int J Environ Res Public Health. (2021) 18:8986. doi: 10.3390/ijerph18178986
8. Ghendov-Mosanu A, Cristea E, Patras A, Sturza R, Padureanu S, Deseatnicova O, et al. Potential application of Hippophae rhamnoides in wheat bread production. Molecules. (2020) 25:1272. doi: 10.3390/molecules25061272
9. Selvamuthukumaran M, Farhath K. Evaluation of shelf stability of antioxidant rich seabuckthorn fruit yoghurt. Int Food Res J. (2014) 21:759–65.
10. Wang H, Sun X, Hua S, Teng X. Historical record and current sistuation of pharmaceutical R&D about seabuckthorn in China. Glob Seabuckthorn Res Dev. (2012) 10:25–8.
11. Suryakumar G, Gupta A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J Ethnopharmacol. (2011) 138:268–78. doi: 10.1016/j.jep.2011.09.024
12. Masoodi K, Wani W, Dar Z, Mansoor S, Anam-ul-Haq S, Farooq I, et al. Sea buckthorn (Hippophae rhamnoides L.) inhibits cellular proliferation, wound healing and decreases expression of prostate specific antigen in prostate cancer cells in vitro. J Funct Foods. (2020) 73:104102. doi: 10.1016/j.jff.2020.104102
13. Olas B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem Toxicol. (2016) 97:199–204. doi: 10.1016/j.fct.2016.09.008
14. Rédei D, Kúsz N, Rafai T, Bogdanov A, Burián K, Csorba A, et al. 14-noreudesmanes and a phenylpropane heterodimer from sea buckthorn berry inhibit Herpes simplex type 2 virus replication. Tetrahedron. (2019) 75:1364–70. doi: 10.1016/j.tet.2019.01.050
15. Tanwar H, Singh D, Singh S, Ganju L. Anti-inflammatory activity of the functional groups present in Hippophae rhamnoides (seabuckthorn) leaf extract. Inflammopharmacology. (2018) 26:291–301. doi: 10.1007/s10787-017-0345-0
16. Verma H, Chahota R, Palial A, Sharma M. Antibacterial properties of seabuckthorn (Hippophae rhamnoides L.) leaf extracts against common skin and wound bacteria. Indian J Vet Res. (2011) 20:38–41.
17. Stobdan T, Chaurasia O, Korekar G, Yadav A, Singh S. Attributes of seabuckthorn (Hippophae rhamnoides L.) to meet nutritional requirements in high altitude. Def Sci J. (2010) 60:226–30. doi: 10.14429/dsj.60.344
18. Pop R, Weesepoel Y, Socaciu C, Pintea A, Vincken J, Gruppen H. Carotenoid composition of berries and leaves from six romanian sea buckthorn (Hippophae rhamnoides L.) varieties. Food Chem. (2014) 147:1–9. doi: 10.1016/j.foodchem.2013.09.083
19. Yang F, Suo Y, Chen D, Tong L. Protection against vascular endothelial dysfunction by polyphenols in sea buckthorn berries in rats with hyperlipidemia. Biosci Trends. (2016) 10:188–96. doi: 10.5582/bst.2016.01056
20. Marsiñach S, Cuenca A. The impact of sea buckthorn oil fatty acids on human health. Lipids Health Dis. (2019) 18:145. doi: 10.1186/s12944-019-1065-9
21. ISA. The annual report of international seabuckthorn development for the year of 2020. Beijing: China WaterPower Press (2021).
22. Government of Ontario. Minist agric food rural aff ont canda. (2022). Available online at: http://www.omafra.gov.on.ca/english/crops/facts/seabuckthorn.htm (accessed May 5, 2022).
23. ChinesePharmacopoeia. Pharmacopoeia of the people’s republic of china 2020. Beijing: China Medical Science Press (2020). 191 p.
26. Hu J. Main achievements of systematic planting and development of seabuckthorn in china in past 35 years (1985~2020). Int J Ecol. (2021) 10:500–8.
27. Ji M, Gong X, Li X, Wang C, Li M. Advanced research on the antioxidant activity and mechanism of polyphenols from hippophae species—a review. Molecules. (2020) 25:917. doi: 10.3390/molecules25040917
28. Teleszko M, Wojdyło A, Rudziñska M, Oszmiañski J, Golis T. Analysis of Lipophilic and Hydrophilic bioactive compounds content in sea buckthorn (Hippophaë rhamnoides L.) berries. J Agric Food Chem. (2015) 63:4120–9. doi: 10.1021/acs.jafc.5b00564
29. Kuhkheil A, Badi H, Mehrafarin A, Abdossi V. Chemical constituents of sea buckthorn (Hippophae rhamnoides L.) fruit in populations of central alborz mountains in Iran. Res J Pharmacogn. (2017) 4:1–12.
30. Saeidi K, Alirezalu A, Akbari Z. Evaluation of chemical constitute, fatty acids and antioxidant activity of the fruit and seed of sea buckthorn (Hippophae rhamnoides L.) grown wild in Iran. Nat Prod Res. (2016) 30:366–8. doi: 10.1080/14786419.2015.1057728
31. Vaitkevièienë N, Jarienë E, Danilèenko H, Kulaitienë J, Mažeika R, Hallmann E, et al. Comparison of mineral and fatty acid composition of wild and cultivated sea buckthorn berries from lithuania. J Elem. (2019) 24:1101–13. doi: 10.5601/jelem.2019.24.1.1759
32. Arif S, Ahmed S, Shah A, Hassan L, Awan S, Hamid A, et al. Determination of optimum harvesting time for vitamin C, oil and mineral elements in berries sea buckthorn (Hippophae rhamnoides). Pak J Bot. (2010) 42:3561–8.
33. Biel W, Jaroszewska A. The nutritional value of leaves of selected berry species. Sci Agric. (2017) 74:405–10. doi: 10.1590/1678-992x-2016-0314
34. Tkacz K, Wojdyło A, Turkiewicz I, Bobak Ł, Nowicka P. Anti-oxidant and anti-enzymatic activities of sea buckthorn (Hippophaë rhamnoides L.) fruits modulated by chemical components. Antioxidants. (2019) 8:618. doi: 10.3390/antiox8120618
35. Ficzek G, Mátravölgyi G, Furulyás D, Rentsendavaa C, Jócsák I, Papp D, et al. Analysis of bioactive compounds of three sea buckthorn cultivars (Hippophaë rhamnoides L. ‘askola’, ‘leikora’, and ‘orangeveja’) with HPLC and spectrophotometric methods. Eur J Hortic Sci. (2019) 84:31–8. doi: 10.17660/eJHS.2019/84.1.5
36. Lee Y, Jang H, Park K, Kim S, Kim J, Kim J, et al. Phytochemical analysis of the fruits of sea buckthorn (Hippophae rhamnoides): identification of organic acid derivatives. Plants. (2021) 10:860. doi: 10.3390/plants10050860
37. Bal L, Meda V, Naik S, Satya S. Sea buckthorn berries: a potential source of valuable nutrients for nutraceuticals and cosmoceuticals. Food Res Int. (2011) 44:1718–27. doi: 10.1016/j.foodres.2011.03.002
38. Odgerel U, Islam M, Kitamura Y, Kokawa M, Odbayar T. Effect of micro wet milling process on particle sizes, antioxidants, organic acids, and specific phenolic compounds of whole sea buckthorn (Hippophae rhamnoides L.) juices. J Food Process Preserv. (2021) 45:1–14. doi: 10.1111/jfpp.15474
39. Ma C, Zheng X, Deng S, Erdake A, He H. Determination and analysis of amino acids in fruits of Hippophae rhamnoide. Protect Forest Sci Technol. (2019) 9:39–40. doi: 10.13601/j.issn.1005-5215.2019.09.013
40. Tan L, Zhao J, Ma J, Ji T, Dong Q, Shen J. Analysis of nutritional compositions and nutritional quality evaluation in different parts of yushu hippophae (Hippophae rhamnoides L. subsp. sinensis). Nat Prod Res Dev. (2018) 30:807–16. doi: 10.16333/j.1001-6880.2018.5.014
41. Eggersdorfer M, Wyss A. Carotenoids in human nutrition and health. Arch Biochem Biophys. (2018) 652:18–26. doi: 10.1016/j.abb.2018.06.001
42. Johnson E. The role of carotenoids in human health. Nutr Clin Care. (2002) 5:56–65. doi: 10.1046/j.1523-5408.2002.00004.x
43. Segliòa D, Krasnova I, Grygier A, Radziejewska−Kubzdela E, Rudziñska M, Górnaś P. Unique bioactive molecule composition of sea buckthorn (Hippophae rhamnoides L.) oils obtained from the peel, pulp, and seeds via physical “solvent−free” approaches. J Am Oil Chem Soc. (2021) 98:1009–20. doi: 10.1002/aocs.12524
44. Dragović-Uzelac V, Bursać Kovačević D, Levaj B, Pedisić S, Mezak M, TomljenovićAna A. Polyphenols and antioxidant capacity in fruits and vegetables common in the croatian diet. Agric Conspec Sci Cus. (2009) 74:175–9.
45. Li N, Hu Y, Ge X. Determination of contents of total flavonoids and total polyphenols in Hippophae rhamnoides L. from different origins and their antioxidant activity. Chem Bioeng. (2021) 38:64–8. doi: 10.3969/j.issn.1672-5425.2021.08.010
46. Zadernowski R, Naczk M, Czaplicki S, Rubinskiene M, Szałkiewicz M. Composition of phenolic acids in sea buckthorn (Hippophae rhamnoides L.) berries. J Am Oil Chem Soc. (2005) 82:175–9. doi: 10.1007/s11746-005-5169-1
47. Ciesarová Z, Murkovic M, Cejpek K, Kreps F, Tobolková B, Koplík R, et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res Int. (2020) 133:109170. doi: 10.1016/j.foodres.2020.109170
48. Guo R, Guo X, Li T, Fu X, Liu R. Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of Sea buckthorn (Hippophaë rhamnoides L.) berries. Food Chem. (2017) 221:997–1003. doi: 10.1016/j.foodchem.2016.11.063
49. Liu S, Xiao P, Kuang Y, Hao J, Huang T, Liu E. Flavonoids from sea buckthorn: a review on phytochemistry, pharmacokinetics and role in metabolic diseases. J Food Biochem. (2021) 45:e13724. doi: 10.1111/jfbc.13724
50. Raudonis R, Raudone L, Janulis V, Viskelis P. Flavonoids in cultivated berries of sea buckthorn (Hippophaë rhamnoides L.). Planta Med. (2014) 80:24. doi: 10.1055/s-0034-1395082
51. Olas B, Kontek B, Malinowska P, Żuchowski J, Stochmal A. Hippophae rhamnoides L. Fruits reduce the oxidative stress in human blood platelets and plasma. Oxid Med Cell Longev. (2016) 2016:1–8. doi: 10.1155/2016/4692486
52. Mohamed E, Tulcan C, Alexa E, Morar D, Dumitrescu E, Muselin F, et al. Sea buckthorn and grape extract might be helpful and sustainable phyto-resources as associated hypolipidemic agents-preliminary study. Sustainability. (2020) 12:9297. doi: 10.3390/su12219297
53. Cho C, Jang H, Lee M, Kang H, Heo H, Kim D. Sea buckthorn (Hippophae rhamnoides L.) leaf extracts protect neuronal PC-12 cells from oxidative stress. J Microbiol Biotechnol. (2017) 27:1257–65. doi: 10.4014/jmb.1704.04033
54. Serban M, Serban A, Ursoniu S, Dragan S. Systematic review on the potential of sea buckthorn Hippophae rhamnoides L. for a possible novel enriched bread for the patients with cardiovascular diseases. Atherosclerosis. (2019) 287:285. doi: 10.1016/j.atherosclerosis.2019.06.882
55. Gęgotek A, Jastrząb A, Jarocka-Karpowicz I, Muszyńska M, Skrzydlewska E. The effect of sea buckthorn (Hippophae rhamnoides L.) seed oil on UV-induced changes in lipid metabolism of human skin cells. Antioxidants. (2018) 7:110. doi: 10.3390/antiox7090110
56. Wu H, Li C, Cui M, Guo H, Chen S, Du J, et al. Polyphenols from Hippophae rhamnoides suppressed colon cancer growth by regulating miRNA-mediated cell cycle arrest and apoptosis in vitro and in vivo. J Funct Foods. (2021) 87:104780. doi: 10.1016/j.jff.2021.104780
57. Kim S, Hwang E, Yi S, Song K, Lee H, Heo T, et al. Sea buckthorn leaf extract inhibits glioma cell growth by reducing reactive oxygen species and promoting apoptosis. Appl Biochem Biotechnol. (2017) 182:1663–74. doi: 10.1007/s12010-017-2425-4
58. Li C, Li J, Li Y, Li L, Luo Y, Li J, et al. Isorhamnetin promotes MKN-45 gastric cancer cell apoptosis by inhibiting PI3K-mediated adaptive autophagy in a hypoxic environment. J Agric Food Chem. (2021) 69:8130–43. doi: 10.1021/acs.jafc.1c02620
59. Rao A, Rao L. Carotenoids and human health. Pharmacol Res. (2007) 55:207–16. doi: 10.1016/j.phrs.2007.01.012
60. Zhou F, Zhang J, Zhao A, Zhang Y, Wang P. Effects of sea buckthorn puree on risk factors of cardiovascular disease in hypercholesterolemia population: a double-blind, randomized, placebo-controlled trial. Anim Biotechnol. (2020) 2020:1–9. doi: 10.1080/10495398.2020.1853139
61. Ntanios F, Duchateau GS. A healthy diet rich in carotenoids is effective in maintaining normal blood carotenoid levels during the daily use of plant sterol-enriched spreads. Int J Vitam Nutr Res. (2002) 72:32–9. doi: 10.1024/0300-9831.72.1.32
62. Basu M, Prasad R, Jayamurthy P, Pal K, Arumughan C, Sawhney R. Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine. (2007) 14:770–7. doi: 10.1016/j.phymed.2007.03.018
63. Guo X, Yang B, Cai W, Li D. Effect of sea buckthorn (Hippophae rhamnoides L.) on blood lipid profiles: a systematic review and meta-analysis from 11 independent randomized controlled trials. Trends Food Sci Technol. (2017) 61:1–10. doi: 10.1016/j.tifs.2016.11.007
64. Yang X, Wang Q, Pang Z, Pan M, Zhang W. Flavonoid-enriched extract from Hippophae rhamnoides seed reduces high fat diet induced obesity, hypertriglyceridemia, and hepatic triglyceride accumulation in C57BL/6 mice. Pharm Biol. (2017) 55:1207–14. doi: 10.1080/13880209.2016.1278454
65. Gao S, Hu G, Li D, Sun M, Mou D. Anti-hyperlipidemia effect of sea buckthorn fruit oil extract through the AMPK and Akt signaling pathway in hamsters. J Funct Foods. (2020) 66:103837. doi: 10.1016/j.jff.2020.103837
66. Ma Z, Sun Q, Chang L, Peng J, Zhang M, Ding X. A natural anti-obesity reagent derived from sea buckthorn polysaccharides: structure characterization and anti-obesity evaluation in vivo. Food Chem. (2022) 375:131884. doi: 10.1016/j.foodchem.2021.131884
67. Guo C, Han L, Li M, Yu L. Seabuckthorn (Hippophaë rhamnoides) freeze-dried powder protects against high-fat diet-induced obesity, lipid metabolism disorders by modulating the gut microbiota of mice. Nutrients. (2020) 12:265. doi: 10.3390/nu12010265
68. Skalski B, Rywaniak J, Szustka A, Żuchowski J, Stochmal A, Olas B. Anti-platelet properties of phenolic and nonpolar fractions isolated from various organs of Elaeagnus rhamnoides (L.) a. nelson in whole blood. Int J Mol Sci. (2021) 22:3282. doi: 10.3390/ijms22063282
69. Skalski B, Stochmal A, Żuchowski J, Grabarczyk Ł, Olas B. Response of blood platelets to phenolic fraction and non-polar fraction from the leaves and twigs of Elaeagnus rhamnoides (L.) a. Nelson in vitro. Biomed Pharmacother. (2020) 124:109897. doi: 10.1016/j.biopha.2020.109897
70. Olas B, Kontek B, Szczesna M, Grabarczyk L, Stochmal A, Zuchowski J. Inhibition of blood platelet adhesion by phenolics’ rich fraction of Hippophae rhamnoides L. fruits. J Physiol Pharmacol. (2017) 68:223–9.
71. Boca A, Ilies R, Saccomanno J, Pop R, Vesa S, Tataru A, et al. Sea buckthorn extract in the treatment of psoriasis. Exp Ther Med. (2019) 17:1020–3. doi: 10.3892/etm.2018.6983
72. Balkrishna A, Sakat S, Joshi K, Joshi K, Sharma V, Ranjan R, et al. Cytokines driven anti-inflammatory and anti-psoriasis like efficacies of nutraceutical sea buckthorn (Hippophae rhamnoides) oil. Front Pharmacol. (2019) 10:1186. doi: 10.3389/fphar.2019.01186
73. Hou D, Di Z, Qi R, Wang H, Zheng S, Hong Y, et al. Sea buckthorn (Hippophaë rhamnoides L.) oil improves atopic dermatitis-like skin lesions via Inhibition of NF-κB and STAT1 activation. Skin Pharmacol Physiol. (2017) 30:268–76. doi: 10.1159/000479528
74. Abdullahzadeh M, Shafiee S. To compare the effect of sea buckthorn and silver sulfadiazine dressing on period of wound healing in patients with second−degree burns: a randomized triple−blind clinical trial. Wound Repair Regen. (2021) 29:732–40. doi: 10.1111/wrr.12916
75. Upadhyay N, Kumar R, Mandotra S, Meena R, Siddiqui M, Sawhney R, et al. Safety and healing efficacy of sea buckthorn (Hippophae rhamnoides L.) seed oil on burn wounds in rats. Food Chem Toxicol. (2009) 47:1146–53. doi: 10.1016/j.fct.2009.02.002
76. Dudau M, Codrici E, Tarcomnicu I, Mihai S, Siddiqui M, Popescu I, et al. A fatty acid fraction purified from sea buckthorn seed oil has regenerative properties on normal skin cells. Front Pharmacol. (2021) 12:737571. doi: 10.3389/fphar.2021.737571
77. Rédei D, Kúsz N, Jedlinszki N, Blazsó G, Zupkó I, Hohmann J. Bioactivity-guided investigation of the anti-inflammatory activity of Hippophae rhamnoides fruits. Planta Med. (2018) 84:26–33. doi: 10.1055/s-0043-114424
78. Zheng W, Bai H, Han S, Bao F, Zhang K, Sun L, et al. Analysis on the constituents of branches, berries, and leaves of Hippophae rhamnoides L. by UHPLC-ESI-QTOF-MS and their anti-inflammatory activities. Nat Prod Commun. (2019) 14:1934578X1987140. doi: 10.1177/1934578X19871404
79. Baek S, Lee D, Jo M, Lee K, Lee Y. Inhibitory effect of 1,5-dimethyl citrate from sea buckthorn (Hippophae rhamnoides) on lipopolysaccharide-induced inflammatory response in RAW 264.7 mouse macrophages. Foods. (2020) 9:269. doi: 10.3390/foods9030269
80. Mulati A, Ma S, Zhang H, Ren B, Zhao B, Wang L, et al. Sea-buckthorn flavonoids alleviate high-fat and high-fructose diet-induced cognitive impairment by inhibiting insulin resistance and neuroinflammation. J Agric Food Chem. (2020) 68:5835–46. doi: 10.1021/acs.jafc.0c00876
81. Shah H, Shakir H, Safi S, Ali A. Hippophae rhamnoides mediate gene expression profiles against keratinocytes infection of Staphylococcus aureus. Mol Biol Rep. (2021) 48:1409–22. doi: 10.1007/s11033-021-06221-3
82. Qadir M, Abbas K, Younus A, Shaikh R. Antibacterial activity of sea buckthorn (Hippophae rhamnoides L.) against methicillin resistant Staphylococcus aureus (MRSA). Pak J Pharm Sci. (2016) 29:1711–3.
83. Smida I, Pentelescu C, Pentelescu O, Sweidan A, Oliviero N, Meuric V, et al. Benefits of sea buckthorn (Hippophae rhamnoides) pulp oil−based mouthwash on oral health. J Appl Microbiol. (2019) 126:1594–605. doi: 10.1111/jam.14210
84. Singh D, Jayashankar B, Mishra K, Tanwar H, Madhusudana S, Belludi A, et al. Adjuvant activity of ethanol extract of Hippophae rhamnoides leaves with inactivated rabies virus antigen. Pharm Biol. (2018) 56:25–31. doi: 10.1080/13880209.2017.1413662
85. Zhang P, Ji H, Hu Q. Research progress in clinical treatment of Alzheimer’s disease and potential drugs from natural products. Acta Pharm Sin. (2022) 2022:1–21. doi: 10.16438/j.0513-4870.2022-0226
86. Dong K, Fernando W, Durham R, Stockmann R, Jayatunga D, Jayasena V. A role of sea buckthorn on Alzheimer’s disease. Int J Food Sci Technol. (2020) 55:3073–81. doi: 10.1111/ijfs.14571
87. Ladol S, Sharma D. The effects of Hippophae rhamnoides in neuroprotection and behavioral alterations against iron-induced epilepsy. Epilepsy Res. (2021) 175:106695. doi: 10.1016/j.eplepsyres.2021.106695
88. Tudor C, Bohn T, Iddir M, Dulf F, Focsan M, Ruginã D, et al. Sea buckthorn 0il as a valuable source of bioaccessible xanthophylls. Nutrients. (2020) 12:76. doi: 10.3390/nu12010076
89. Elvira-Torales L, García-Alonso J, Periago-Castón M. Nutritional importance of carotenoids and their effect on liver health: a review. Antioxidants. (2019) 8:229. doi: 10.3390/antiox8070229
90. Guo Z, Cheng J, Zheng L, Xu W, Xie Y. Mechanochemical-assisted extraction and hepatoprotective activity research of flavonoids from sea buckthorn (Hippophaë rhamnoides L.) pomaces. Molecules. (2021) 26:7615. doi: 10.3390/molecules26247615
91. Ran B, Guo C, Li W, Li W, Wang Q, Qian J, et al. Sea buckthorn (Hippophae rhamnoides L.) fermentation liquid protects against alcoholic liver disease linked to regulation of liver metabolome and the abundance of gut microbiota. J Sci Food Agric. (2021) 101:2846–54. doi: 10.1002/jsfa.10915
92. Zhang G, Liu Y, Liu P. Active components from sea buckthorn (Hippophae rhamnoides L.) regulate hepatic stellate cell activation and liver fibrogenesis. J Agric Food Chem. (2018) 66:12257–64. doi: 10.1021/acs.jafc.8b05306
93. Hao Y, Zhou F, Dong J, Wang Y, Lang Z, Li S, et al. Study on the role of flavonoids derived extract from seed residues of hippophae rhamnoides on high-fat diet induced obese mice. J King Saud Univ Sci. (2020) 32:1597–603. doi: 10.1016/j.jksus.2019.12.017
94. Ma C, Du L, Cai G. Investigation and research on the distribution of seabuckthorn germplasm resources in changji prefecture. Mod Agric Sci Technol. (2022) 2022:139–41. doi: 10.3969/j.issn.1007-5739.2022.02.046
95. Kozhakhiyeva M, Dragoev S, Uzakov Y, Nurgazezova A. Improving the oxidative stability and quality of new functional horse meat delicacy enriched with sea buckthorn (Hippophae rhamnoides) fruit powder extract or seed kernel pumpkin (Cucurbita pero L.) flour. Comptes Rendus L’Academie Bulg Sci. (2018) 70:132–40. doi: 10.7546/CRABS.2018.01.18
96. Salejda A, Nawirska-Olszańska A, Janiewicz U, Krasnowska G. Effects on quality properties of pork sausages enriched with sea buckthorn (Hippophae rhamnoides L.). J Food Qual. (2017) 2017:1–7. doi: 10.1155/2017/7123960
97. Tzachristas A, Pasvanka K, Liouni M, Calokerinos A, Tataridis P, Proestos C. Effect of Hippophae rhamnoides L. leaves treatment on the antioxidant capacity, total phenol content and sensory profile of moschofilero wines vinified with and without added sulphites. Appl Sci. (2020) 10:3444. doi: 10.3390/app10103444
98. Haq S, Mir M, Lone S, Banoo A, Shafi F, Mir S, et al. Explicating genetic diversity based on ITS characterization and determination of antioxidant potential in sea buckthorn (Hippophae spp.). Mol Biol Rep. (2022) 49:5229–40. doi: 10.1007/s11033-021-06619-z
99. Lele V, Monstaviciute E, Varinauskaite I, Peckaityte G, Paskeviciute L, Plytnikaite M, et al. Sea buckthorn (Hippophae rhamnoides L.) and quince (Cydonia oblonga L.) juices and their by-products as ingredients showing antimicrobial and antioxidant properties for chewing candy: nutraceutical formulations. J Food Qual. (2018) 2018:1–8. doi: 10.1155/2018/3474202
100. Kashyap P, Kumar S, Singh D. Performance of antifreeze protein HrCHI4 from Hippophae rhamnoides in improving the structure and freshness of green beans upon cryopreservation. Food Chem. (2020) 320:126599. doi: 10.1016/j.foodchem.2020.126599
101. Gu Y, Chen Z, Fu L. Development of yogurt mixed with sea buckthorn and carrot. China Brew. (2008) 12:113–8.
102. Gu Y, Chen X, Fu L. Preparation of yogurt mixed with Hippophae rhamnoides and tomoto. China Brew. (2008) 29:66–8.
103. Liu H, Wang R, Gao Z. Study on the process of compound yoghurt with sea buckthorn and water chestnut. J Shanxi Datong Univ. (2019) 35:59–62.
104. Nistor O, Bolea C, Andronoiu D, Cotârleţ M, Stănciuc N. Attempts for developing novel sugar-based and sugar-free sea buckthorn marmalades. Molecules. (2021) 26:3073. doi: 10.3390/molecules26113073
105. Yuan L, Liu J, Wu T, Wu H, Liu L. Optimization of technology of Elaeagnus angustifolia and Hippophae rhamnoides compound jam by response surface methodology and prediction of storage period. Storage Proc. (2022) 22:37–44. doi: 10.3969/j.issn.1009-6221.2022.07.006
106. Sun L, Xu X. Preferred research on technology and materials of sea buckthorn composite jam. Storage Proc. (2014) 20:52–4.
107. Selvamuthukumaran M, Khanum F, Bawa A. Development of sea buckthorn mixed fruit jelly. Int J Food Sci Technol. (2007) 42:403–10. doi: 10.1111/j.1365-2621.2006.01233.x
108. Gâtlan A, Gutt G, Naghiu A. Capitalization of sea buckthorn waste by fermentation: optimization of industrial process of obtaining a novel refreshing drink. J Food Proc Pres. (2020) 44:14565. doi: 10.1111/jfpp.14565
109. Xin Y, Zhao S, Yang J, Zhang T, Zhang J, Wang Y. Effect of microwave-drying on the quality and antioxidant properties of Ganoderma lucidum fermented sea-buckthorn tea. Int J Food Eng. (2021) 17:65–74. doi: 10.1515/ijfe-2019-0271
110. Wen P, Zhao P, Qin G, Tang S, Li B, Zhang J, et al. Genotoxicity and teratogenicity of seabuckthorn (Hippophae rhamnoides L.) berry oil. Drug Chem Toxicol. (2020) 43:391–7. doi: 10.1080/01480545.2018.1497047
111. Tulsawani R. Ninty day repeated gavage administration of Hipphophae rhamnoides extract in rats. Food Chem Toxicol. (2010) 48:2483–9. doi: 10.1016/j.fct.2010.06.018
Keywords: sea buckthorn, phytochemistry, nutrients, health benefits, food applications
Citation: Wang Z, Zhao F, Wei P, Chai X, Hou G and Meng Q (2022) Phytochemistry, health benefits, and food applications of sea buckthorn (Hippophae rhamnoides L.): A comprehensive review. Front. Nutr. 9:1036295. doi: 10.3389/fnut.2022.1036295
Received: 04 September 2022; Accepted: 22 November 2022;
Published: 06 December 2022.
Edited by:
Wenjie Sui, Tianjin University of Science and Technology, ChinaReviewed by:
Paucean Adriana, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaDmitriy Shcherbakov, Altai State University, Russia
Khalid Z. Masoodi, Sher-e-Kashmir University of Agricultural Sciences and Technology, India
Songfeng Diao, Chinese Academy of Forestry, China
Copyright © 2022 Wang, Zhao, Wei, Chai, Hou and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyun Chai, chaixy1207@smmu.edu.cn; Guige Hou, houyun820424@bzmc.edu.cn; Qingguo Meng, qinggmeng@ytu.edu.cn
 Zhen Wang
Zhen Wang Fenglan Zhao1
Fenglan Zhao1