The effect of the oak powdery mildew, oak lace bug, and other foliofagous insects on the growth of young pedunculate oak trees
- 1Faculty of Forestry, Department of Forestry and Nature Protection, Chair of Forest Protection, University of Belgrade, Belgrade, Serbia
- 2Faculty of Forestry, Department of Forestry and Nature Protection, Chair of Silviculture, University of Belgrade, Belgrade, Serbia
Pedunculate oak (Quercus robur L., 1753) is one of the widely distributed oak species in Europe. A large number of organisms develop on its leaves. To determine the extent to which the oak powdery mildew, oak lace bug, and other foliofagous insects affect the growth of young oak trees, three experimental fields were selected in a 10-year-old pedunculate oak stand. In each of them, 50 trees were randomly selected, and their height was measured at the beginning of the vegetative season. The first experimental field was treated with a systemic insecticide, the second with a systemic fungicide, and the third, a comparison area, with water, during the entire vegetative season. At the end of the vegetative season, 25 plants with one apical branch were selected in each experimental field. Their height was measured, and 20 leaves were taken from each plant to determine the extent of the damage on them at the end of the experiment. After processing the obtained data, it was determined that: 1. Both foliofagous insects and oak leaf inhabiting fungi affect the growth of the oak trees significantly; 2. The oak lace bug did not influence the growth of the young trees significantly, as its abundance was low in all of the experimental areas; 3. The greatest damage on the leaves was caused by defoliator insects, which is why they contributed the most to the decrease in growth caused by insects; 4. The influence of the foliofagous insects on the growth of the trees was not significantly different from the influence of fungi; 5. Suppression of oak powdery mildew and foliofagous insects on young trees is useful as it positively influences the vitality and growth of those trees, and contributes to economic and ecological gain.
1 Introduction
Pedunculate oak (Quercus robur L., 1753) is one of the most widely distributed oak species in Europe (Bobinac et al., 2012; Puchałka et al., 2017). Its range covers most of Europe, excluding its most southern and northern parts (Eaton et al., 2016). This species always had significant meaning for the people, as it provided construction and fuel material, food for livestock, and bark for tanning (Eaton et al., 2016). Because of its impressive appearance and longevity, it has a symbolic role in many cultures in Europe (Askeyev et al., 2005; Mills, 2013; Eaton et al., 2016). Its forests have a significant ecological value as they provide high biodiversity (Mölder et al., 2019). Due to its high quality of wood, it is one of the most important species in managed forests in Europe (Eaton et al., 2016).
A large number of organisms develop on pedunculate oak leaves (Karadžić, 2010; Dobrosavljević et al., 2020; Ermolaev et al., 2021; Mladenović et al., 2021; Marković, 2022). Among them, the oak lace bug - Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae) and oak powdery mildew, which is most frequently caused by Erysiphe alphitoides (Griffon and Maubl). Braun and Takam (Erysiphales: Erysiphaceae), present the most problematic ones in southeastern Europe (Glavaš, 2011; Pap et al., 2013; Simov et al., 2018; Drekić et al., 2019; Bălăcenoiu et al., 2021a; Franjević et al., 2023). Defoliators such as Lymantria dispar Linnaeus, 1758 (Lepidoptera: Erebidae), Tortrix viridana Linnaeus, 1758 (Lepidoptera: Tortricidae), Erannis defoliaria (Clerck, 1759) and Operophtera brumata (Linnaeus, 1758) (Lepidoptera: Geometridae) can also cause significant damage as their outbreaks can spread over large areas (Marović et al., 1998; Harapin and Jurc, 2000; Pernek et al., 2008; Tomescu and Netoiu, 2008).
Corythucha arcuata and Erysiphe alphitoides are invasive species’ (Marçais and Desprez-Loustau, 2014; Bălăcenoiu et al., 2021a). The first one originates from North America (Csóka et al., 2020), while the origin of second one is most probably from Asia (Desprez-Loustau et al., 2017). The first finding of C. arcuata in Europe happened in Italy in 2000 (Bernardinelli and Zandigiacomo, 2000), while E. alphitoides was first found in France in 1907 (Hariot, 1907). These two species are now one of the most widely distributed oak leaf-inhabiting pest organisms in Europe (Marçais and Desprez-Loustau, 2014; Csóka et al., 2020). C. arcuata causes significant damage during each vegetative season. Severe outbreaks of this species have been reported in many European countries (Paulin et al., 2020). Its larvae and adults damage the leaves by sucking the sap on the underside of the leaf. Necroses which their feeding causes can cover the entire leaf area in the case of high abundance. That is why decolorization, lower photosynthetic activity, transpiration, and stomatal conductance occur on those plants (Nikolic et al., 2019; Paulin et al., 2020; Bălăcenoiu et al., 2021a). All of these effects can consequentially lead to a decrease in growth, premature leaf abscission, and a decrease in the size of the acorn (Tomescu et al., 2018; Drekić et al., 2019; Paulin et al., 2020). E. alphitoides is constantly present in oak forests. This obligate parasite creates an epiphyte mycelium on the leaf, which takes nutrients from the host and covers the leaf surface (Karadžić and Milijašević, 2005). All this consequentially causes a reduction in photosynthetic activity and transpiration (Pap et al., 2014b). That causes a decrease in growth and can cause dieback of younger plants (Karadžić and Milijašević, 2005; Bert et al., 2016). The dieback of young oak trees which this fungus causes is a significant problem (Karadžić and Milijašević, 2005; Pap et al., 2012). That is why the control of this pathogen is conducted during forest regeneration (Bobinac and Karadžić, 1994; Glavaš, 2011; Pap et al., 2012). E. alphitoides causes problems even in older forests in the cases of defoliation, when it can significantly diminish the vitality of oak trees (Pap et al., 2014b).
As E. alphitoides has been present in Europe for more than 100 years, a lot is known about it (Desprez-Loustau et al., 2011; Marçais and Desprez-Loustau, 2014; Lonsdale, 2015; Kebert et al., 2022; Mieslerová et al., 2022). L. dispar, T. viridana, E. defoliaria, and O. brumata have also been a topic of many studies (Ivashov et al., 2002; Tikkanen and Julkunen-Tiitto, 2003; Glavendekić, 2010; Milanović et al., 2020a,b, 2022). C. arcuata is still a new species for Europe so it is currently intensively studied (Bernardinelli, 2006; Franjević et al., 2018; Drekić et al., 2019; Nikolic et al., 2019; Csóka et al., 2020; Kern et al., 2021; Marković et al., 2021a; Bălăcenoiu et al., 2021b; Paulin et al., 2023; Stancă-Moise et al., 2023; Valdés-Correcher et al., 2023). As pedunculate oak is one of the most significant European oaks (Eaton et al., 2016; Mölder et al., 2019) we conducted a study to determine: how C. arcuata and other foliophagous insects affect the growth of young pedunculate oak trees; which type of foliofagous insect damage is dominant on the leaves; how oak powdery mildew affects the growth of young trees; and does the influence of foliofagous insect on the growth of young oak trees differ from the influence of oak powdery mildew.
2 Materials and methods
2.1 Study area
The study was conducted in 2022 in a 10-year-old regenerated pedunculate oak stand1 (44° 45′ 2.88˝ N and 19° 59′ 45.88˝ E). It is located in a plane, at an altitude of 74 m. The average tree height was 2.4 m and the average diameter at root collar was 2.8 cm. The studied area is located on an alluvial deposit of clay and sand, where the soil is eutric cambisol. The average annual temperature is about 11°C, while the annual precipitation is 569.6 mm. The climate is characterized as continental with some features of the Pannonian-steppe temperate continental climate. The plants in the investigated stand use only atmospheric water and underground water in spring since the area is not flood-prone.
2.2 Experimental design
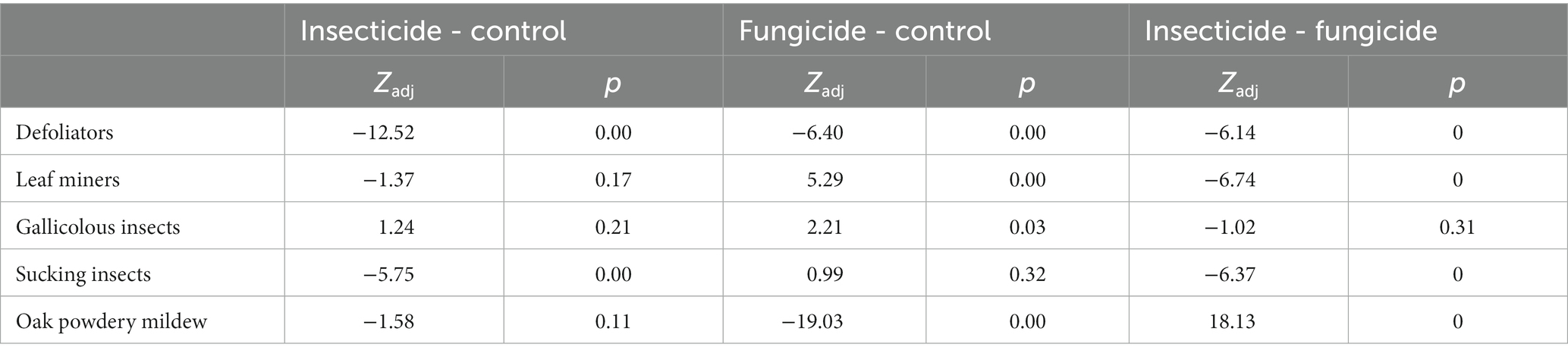
Three experimental areas, measuring 20 × 10 m, separated by a distance of 100 m, were selected in the stand. At each of them, 50 randomly selected trees were singled out at the beginning of the study (on April 1st). They were labeled and their heights were measured by a tape measure with a precision of 1 cm. The first study area was treated with the systemic insecticide Tonus (active substance Acetamiprid 200 g/kg) in a concentration of 0.25 g per liter of water to prevent and suppress insect damage. The second area was treated with the systemic fungicide Falcon 460-EC (Tebuconazole 167 g/L + Triadimenol 43 g/L + Spiroxamine 250 g/L, in a concentration of 0.35 mL) per liter of water to prevent and suppress the harmful fungi. The third area was the comparison area which was treated only with water. These pesticides were selected because they have a broad spectrum of effect and were already successfully used or they gave satisfactory results in similar experiments (Pap et al., 2015; Drekić et al., 2021). The pesticides were applied by spraying from the ground with a backpack sprayer. Each experimental area was treated with 10 liters of the listed formulation prepared with water (500 L/ha). All of the areas were treated simultaneously, every 15 days starting from April 1 to October 1 (entire vegetation). After that, 25 plants with a single apex and similar initial heights (± 25 cm) were selected in each experimental field to isolate extreme values, as some plants were broken while some formed multiple apical branches. Their heights were measured on the 15th of October when the experiment was finished. Twenty leaves were then randomly selected from each of the plants to assess the amount of damage caused by the analyzed organisms. They were packed in plastic bags and brought to the laboratory of the University of Belgrade Faculty of Forestry, where they were kept in the refrigerator for 2 days until the analyses were done. Damages were divided into the following groups: defoliators, miners, gallers, sucking insects, and oak powdery mildew (Figure 1). The assessment of the damage by category was done visually by the naked eye. The damaged area was estimated as the share of leaf area covered by mycelia, mines, galls, discoloration or simply eaten (missing) in relation to the total surface area of the leaf. The damaged area was measured in percentages as a relative measure because the leaf size differed significantly between and within each tree.
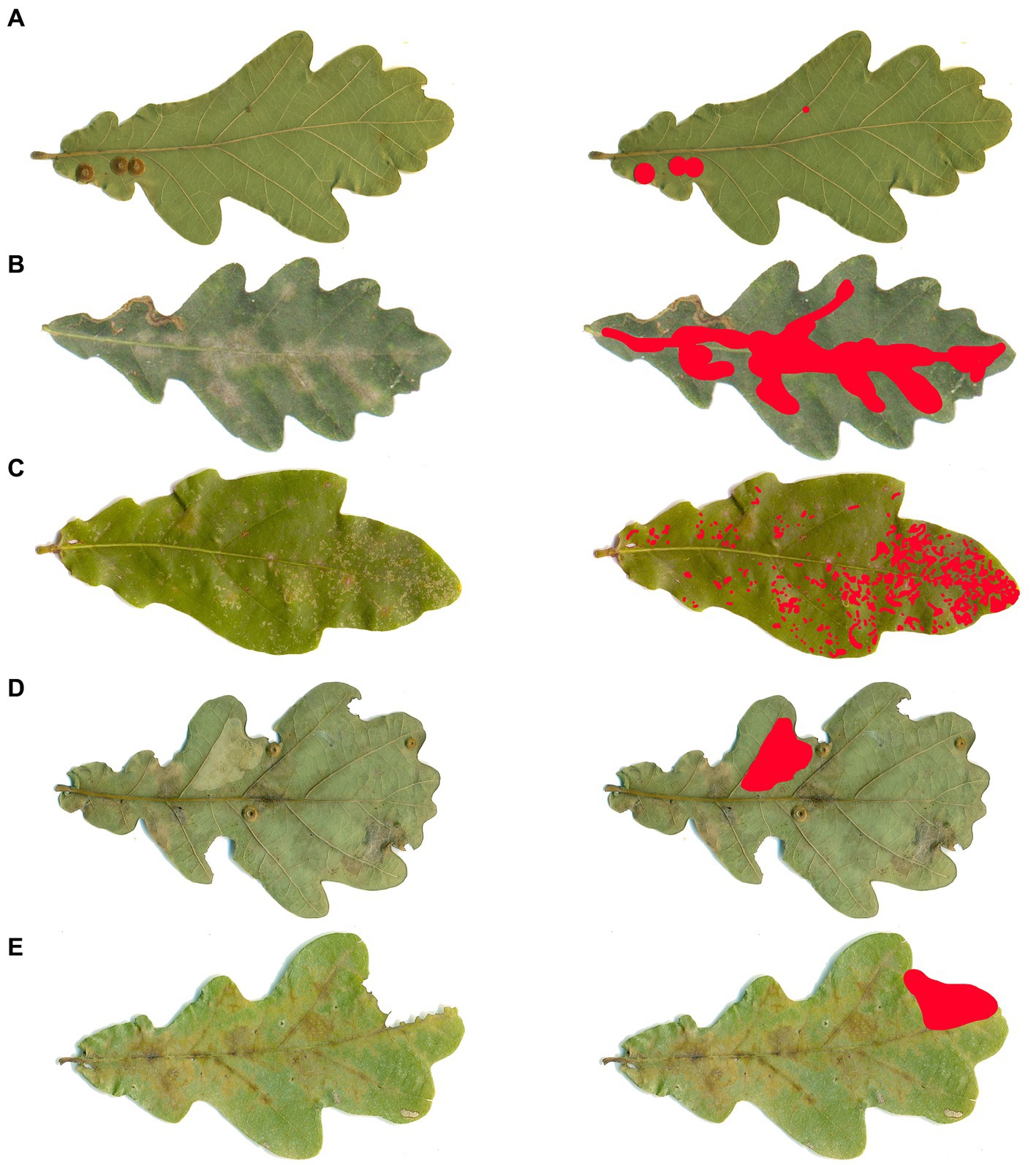
Figure 1. Illustration of how the different damage types were identified and estimated: (A) galls (Cynipidae sp.); (B) oak powdery mildew; (C) oak lace bug (Corythucha arcuata); (D) mines (Phyllonorycter sp.); (E) defoliation.
2.3 Leaf damaging organisms
Before each treatment and at the end of the experiment, leaves from randomly selected plants in the comparison area were analyzed to identify the foliofagous insect fauna on them. The noted species were identified on the site as they are common for the area in which the study was conducted. As oak powdery mildew can be caused by multiple fungi (Karadžić and Milijašević, 2005) of which E. alphitoides is listed as the most important one in the studied area, the damage on the leaves was labeled only as oak powdery mildew.
2.4 Statistical analysis
As the distribution of the analyzed parameters did not fit any of the standardized distributions (Kolmogorov–Smirnov test), nonparametric tests were used for further analysis. Kruskal-Wallis ANOVA by Ranks was used to determine the influence of the treatments on the growth of the analyzed trees and the influence of the treatments on the damage caused by different insect groups. Mann–Whitney U test was used as a post-hoc test, to determine the differences between individual treatments where the Kruskal-Wallis ANOVA showed significant differences. Mann–Whitney U test was also used to determine the differences between the leaf areas damaged by different insect groups in each treatment. All the data were analyzed at the tree level, at the level of significance 0.05. All of the statistical analyses were performed using Statistica 8.0 (StatSoft, Inc., Tulsa, OK, United States).
3 Results
Among the insects observed on the experimental areas, the most significant sucking species was C. arcuata; defoliators L. dispar, E. defoliaria, O. brumata T. viridana and Periclista sp. (Hymenoptera, Tenthredinidae); gallers Andricus curvator Hartig, 1840, Neuroterus numismalis (Fourcroy, 1785) and N. quercusbaccarum (Linnaeus, 1758) (Hymenoptera, Cynipidae); miners Profenusa pygmaea (Klug, 1816) (Hymenoptera, Tenthredinidae), Phyllonorycter harrisella (Linnaeus, 1761), Ph. roboris (Lepidoptera, Gracillariidae), and Tischeria ekebladella (Bjerkander, 1795) (Lepidoptera, Tischeriidae). The dominant fungal damage on the leaves was caused by oak powdery mildew.
Statistically significant differences were identified between the treated and the comparison area in the intensity of the damage caused by oak powdery mildew, defoliator insects, sucking insects, and leaf miners (Figure 2; Table 1). No significant differences were observed in the damage caused by gallicolous insects. In the area treated with fungicide, the intensity of the damage caused by leaf miners and sucking insects was significantly higher compared to the comparison area.
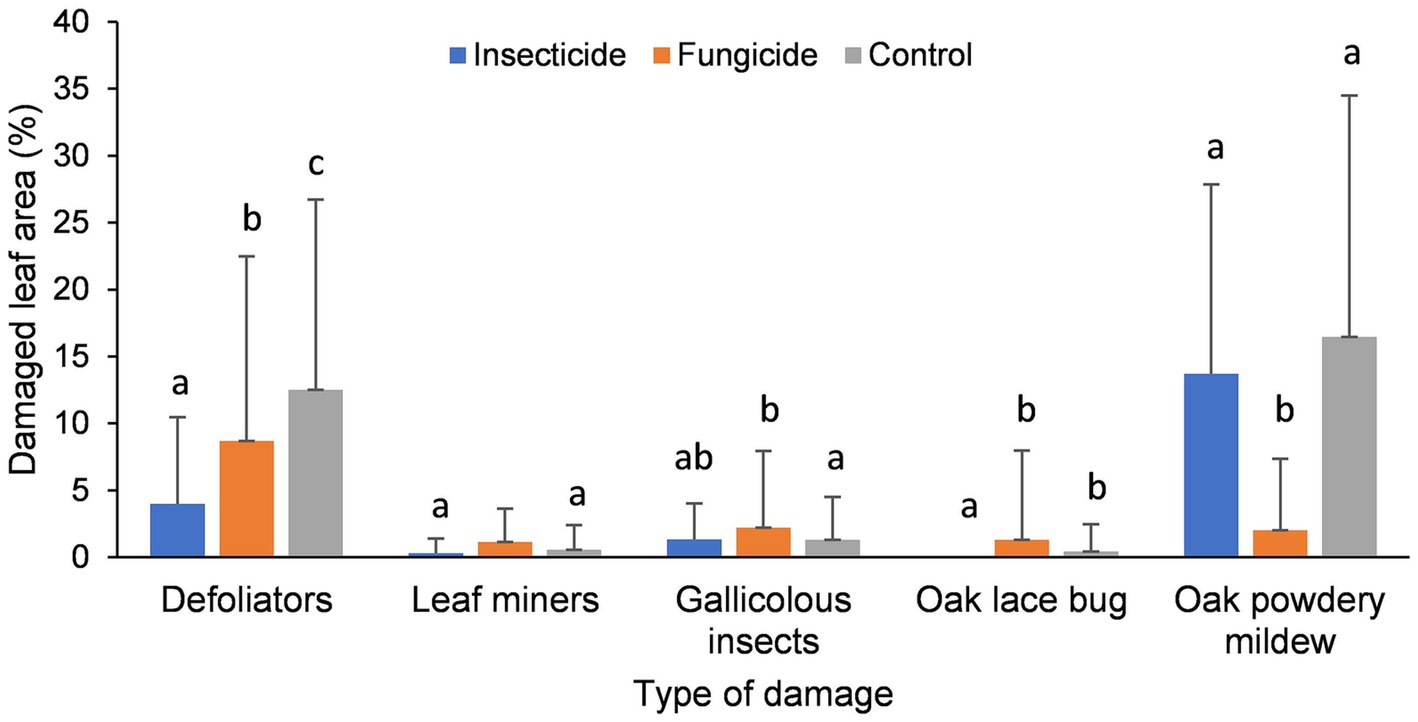
Figure 2. Damaged leaf area per type of damage (%) + SD, per each treatment, with letters above indicating the significant differences.
Statistically significant differences in growth were identified between each of the treated and the comparison area (Insecticide - Zadj = 4.590, p = 0.000; Fungicide - Zadj =4.910, p = 0.000). There were no significant differences between the treated areas (Zadj = −1.136, p = 0.256). The greatest increase in growth was detected in the area treated with fungicide, less with insecticide and the lowest was in the comparison area (Figure 3). The growth on the area treated with fungicide was on average 62.3% higher, and on the surface treated with insecticide 50.5% higher in respect to the comparison area.
Figure 3. Influence of pesticide treatment on the height growth of the analyzed trees (cm) ± SD, with letters above indicating the significant differences.
4 Discussion
Pedunculate oak hosts a large number of insects and fungi (Županić et al., 2009; Marković and Stojanović, 2011; Wrzesińska, 2017; Demeter et al., 2021; Ermolaev et al., 2021; Milanović et al., 2021; Jankowiak et al., 2022; Pilipović et al., 2022; Marković and Dobrosavljević, 2023). Many of them can be effectively suppressed by using insecticides and fungicides (Mihajlović and Glavendekić, 2006; Margaletić et al., 2007; Glavaš, 2011; Pap et al., 2012, 2014a; Pajnik et al., 2017; Drekić et al., 2021). The results of our study showed that the insecticide applied in the experiment can be successfully used for the control of sucking insects, defoliators, and leaf-mining insects, as the leaf area damaged by these groups was significantly lower in the insecticide-treated area. It should not be used to control gallicolous insects because it is not very effective. The applied fungicide can be used to efficiently control the oak powdery mildew as the damage caused by it was significantly lower in the fungicide-treated area.
Among the fungi, oak powdery mildew caused the greatest damage on the observed leaves on the area treated with insecticide, and the comparison area. This was expected because oak powdery mildew is one of the biggest problems on the leaves of young pedunculate oak trees in Southeastern Europe (Karadžić and Milijašević, 2005; Glavaš, 2011; Pap et al., 2013). The plants treated with fungicide showed lower oak mildew damage in comparison to other plots, and also the greatest height increment. This height increment is most likely connected to the lower share of damaged leaf area and subsequentially more available leaf area for photosynthesis (Nikolic et al., 2019; Paulin et al., 2020; Bălăcenoiu et al., 2021a). Of the insect groups, defoliators damaged the greatest leaf area. Damage from sucking insects, leaf miners, and gallicolous insects was negligible on all three plots. The fact that the damage from the sucking insects was small is a real surprise because, among the foliophagous insects in the old pedunculate oak forest near the location where the study was carried out, significant discoloration in the leaves caused by C. arcuata was noticed. Since the discoloration of the leaves in the old forest was higher, C. arcuata may prefer older trees, as it is already known that insect community and abundance change with the forest ages (Nagy et al., 2016; Marković et al., 2021b). The reason for this may be the fact that the allocation of defense chemicals is highest in young trees. On the other side, mature trees require resources for flower and seed production, they are frequently water deficient and have unfavorable photosynthesis/respiration, and saplings need the energy for the production of more aboveground biomass and increase of photosynthetic area, so they have a significantly lower amount of defense chemicals (Boege and Marquis, 2005; Barton and Hanley, 2013). In the forest where the research was carried out, areas with young trees of pedunculate oak, Turkey oak (Q. cerris L.), and Hungarian oak (Q. frainetto Ten.) of similar age were observed. The damage caused by C. arcuata was significantly greater on Turkey and Hungarian oak than on pedunculate oak. The pedunculate oak may be a less favorable host for C. arcuata compared to other oak species (Marković et al., 2021a).
There were no significant differences in tree growth between the treated areas. This shows that insects and fungi have a similar effect on their growth. This result is a novelty since the literature only mentions the effect of the oak powdery mildew (Bobinac and Karadžić, 1994; Karadžić and Milijašević, 2005; Glavaš, 2011; Pap et al., 2013; Rađević et al., 2020). In the area treated with the fungicide, the damage from sucking insects and leaf miners was higher than in the comparison area. Since oak powdery mildew was suppressed on it, this higher abundance indicates that there are competitive relationships between them. Such a relationship between the oak powdery mildew and insects is already known (Zargaran et al., 2012; Marković et al., 2021a).
The results of this study show that during the growing season, under the influence of fungi, the height growth of 10-year-old pedunculate oak trees decreases by 62.3%, and under the influence of insects by 50.5%. The real growth decrease is greater since the pesticides used did not achieve complete protection of the leaves from insects and fungi. The influence caused by these organisms is significant, which is why the need to suppress them arises. In plants up to 2 years of age, the control of oak powdery mildew should be carried out, as it is one of the limiting factors of the plants’ development (Glavaš, 2011; Pap et al., 2012). Pesticide treatment of young trees older than 2 years is also useful as it positively influences their height growth and vitality. However, the treatment of older trees is complicated because of the characteristics of those stands (high density and height of trees), so the question of cost to benefit arises. The benefits of increased growth contribute both to the ecological functions such as sequestration of carbon dioxide, and economic functions such as the production of more wood. On the other side, any pesticide treatment affects other, non-targeted organisms, so the balance between these two needs to be found. The only place where the suppression of pest organisms on older trees should be carried out is in parks, gardens, and other areas where it does not require the use of expensive techniques and does not cause serious non-target effects.
Pedunculate oak is one of the dominant forest-forming species throughout Europe (Eaton et al., 2016). The restoration of its forests encounters many problems (Rumiantsev et al., 2018; Axer et al., 2023). To assist it, it is important to have a broader knowledge of the factors that can negatively affect those forests. When talking about the influence of insects and fungi on the growth of its young trees, based on the results of this study, it can be concluded that: 1. Both foliofagous insects and fungi significantly affect the height growth of the pedunculate oak, as the trees treated with pesticides had less damaged leaf area and grew significantly higher than trees in the comparison area; 2. C. arcuata did not influence the growth of the young trees significantly, as its abundance was low in all of the experimental areas; 3. The greatest damage on the leaves was caused by defoliator insects, which is why they contributed the most to the decrease in growth caused by insects; 4. The influence of the foliofagous insects on the growth of the trees was not significantly different from the influence of the fungi; 5. Suppression of oak powdery mildew and foliofagous insects on young trees is useful as it positively influences the vitality and growth of those trees, and contributes to economic and ecological gain; 6. As pedunculate oak is a less favorable host for C. arcuata compared to other oak species, it would be useful to determine whether there are differences between it and other oak species in terms of the influence of oak lace bug on the growth of young trees.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Ethics board of Faculty of Forestry, University of Belgrade. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ČM: Conceptualization, Investigation, Resources, Supervision, Writing – original draft, Writing – review & editing. BK: Data curation, Investigation, Resources, Writing – review & editing. UP: Investigation, Writing – review & editing. JD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Serbian Ministry of Education, Science, and Technological Development, Grant Number 451–03-9/2022–14/200169 for financing scientific research at the Faculty of Forestry University of Belgrade.
Acknowledgments
We would like to thank the Public Company “Vojvodinašume,” from Petrovaradin, Serbia, for the help provided during the fieldwork.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^All the data except the plant dimensions were gathered from the Public company “Vojvodinašume” which manages the forests in which the experiment was conducted.
References
Askeyev, O. V., Tischin, D., Sparks, T. H., and Askeyev, I. V. (2005). The effect of climate on the phenology, acorn crop and radial increment of pedunculate oak (Quercus robur) in the middle Volga region, Tatarstan. Russia. Int. J. Biometeorol. 49, 262–266. doi: 10.1007/s00484-004-0233-3
Axer, M., Martens, S., Schlicht, R., Eisenhauer, D., and Wagner, S. (2023). Modelling natural regeneration of oak in Saxony, Germany: identifying factors influencing the occurrence and density of regeneration. IForest 16, 47–52. doi: 10.3832/ifor4064-015
Bălăcenoiu, F., Nețoiu, C., Tomescu, R., Simon, D. C., Buzatu, A., Toma, D., et al. (2021a). Chemical control of Corythucha arcuata (Say, 1832), an invasive alien species, in oak forests. Forests 12:770. doi: 10.3390/f12060770
Bălăcenoiu, F., Simon, D. C., Nețoiu, C., Toma, D., and Petrițan, I. C. (2021b). The seasonal population dynamics of Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae) and the relationship between meteorological factors and the diurnal flight intensity of the adults in Romanian oak forests. Forests 12:1774. doi: 10.3390/f12121774
Barton, K. E., and Hanley, M. E. (2013). Seedling-herbivore interactions: insights into plant defence and regeneration patterns. Ann. Bot. 112, 643–650. doi: 10.1093/aob/mct139
Bernardinelli, I. (2006). Potential host plants of Corythucha arcuata (Het., Tingidae) in Europe: a laboratory study. J. Appl. Entomol. 130, 480–484. doi: 10.1111/j.1439-0418.2006.01098.x
Bernardinelli, I., and Zandigiacomo, P. (2000). Prima segnalazione di Corythucha arcuata (say) Heteroptera, Tingidae in Europa. Inf. Fitopatol. 50, 47–49.
Bert, D., Lasnier, J.-B., Capdevielle, X., Dugravot, A., and Desprez-Loustau, M.-L. (2016). Powdery mildew decreases the radial growth of Oak trees with cumulative and delayed effects over years. PLOS ONE 11:e0155344. doi: 10.1371/journal.pone.0155344
Bobinac, M., Batos, B., Miljkovic, D., and Radulovic, S. (2012). Polycyclism and phenological variability in the common oak (Quercus robur L.). Arch. Biol. Sci. 64, 97–105. doi: 10.2298/ABS1201097B
Bobinac, M., and Karadžić, D. (1994). Zaštita ponika lužnjaka (Q. robur L.) od hrastove pepelnice (Microsphaera alphitoides Griff. et Maubl.) - mere za smanjenje rizika semene obnove. Proceedings of conference Zaštita bilja juče, danas, sutra. eds. M. Šestović, N. Nešković, and I. Perić (Vrnjačka Banja, Serbia: Društvo za zaštitu bilja Srbije), 617–627.
Boege, K., and Marquis, R. J. (2005). Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends Ecol. Evol. 20, 441–448. doi: 10.1016/j.tree.2005.05.001
Csóka, G., Hirka, A., Mutun, S., Glavendekić, M., Mikó, Á., Szőcs, L., et al. (2020). Spread and potential host range of the invasive oak lace bug [Corythucha arcuata (Say, 1832) – Heteroptera: Tingidae] in Eurasia. Agric. For. Entomol. 22, 61–74. doi: 10.1111/afe.12362
Demeter, L., Molnár, Á. P., Öllerer, K., Csóka, G., Kiš, A., Vadász, C., et al. (2021). Rethinking the natural regeneration failure of pedunculate oak: the pathogen mildew hypothesis. Biol. Conserv. 253:108928. doi: 10.1016/j.biocon.2020.108928
Desprez-Loustau, M.-L., Feau, N., Mougou-Hamdane, A., and Dutech, C. (2011). Interspecific and intraspecific diversity in oak powdery mildews in Europe: coevolution history and adaptation to their hosts. Mycoscience 52, 165–173. doi: 10.1007/S10267-010-0100-5
Desprez-Loustau, M.-L., Massot, M., Feau, N., Fort, T., de Vicente, A., Torés, J. A., et al. (2017). Further support of Conspecificity of oak and mango powdery mildew and first report of Erysiphe quercicola and Erysiphe alphitoides on mango in mainland Europe. Plant Dis. 101, 1086–1093. doi: 10.1094/PDIS-01-17-0116-RE
Dobrosavljević, J., Marković, Č., Marjanović, M., and Milanović, S. (2020). Pedunculate oak leaf miners’ community: urban vs. Rural Habitat. Forests 11:1300. doi: 10.3390/f11121300
Drekić, M., Poljaković-Pajnik, L., Milović, M., Kovačević, B., Pilipović, A., and Pap, P. (2021). Efficacy of some insecticides for control of oak lace bug (Corythucha arcuata say). Poplar 21–26, 21–26. doi: 10.5937/topola2108021D
Drekić, M., Poljaković-Pajnik, L., Pilipović, A., Nikolić, N., Poljaković Pajnik, L., Pilipović, A., et al. (2019). Supression of oak lace bug Corytucha arcuata say. Šumarstvo. 3–4, 215–224.
Eaton, E., Caudullo, G., Oliveira, S., and de Rigo, D. (2016). “Quercus robur and Quercus petraea in Europe: distribution, habitat, usage and threats,” in European atlas of Forest tree species, eds. J. San-Miguel-Ayanz, D. Rigode, G. Caudullo, T. Houston Durrant, and A. Mauri (Publication Office of the European Union, Luxembourg), 160–163.
Ermolaev, I. V., Ponomarev, V. I., Vasil’ev, A. A., and Kumaeva, M. S. (2021). Phytophagous insects associated with the Pedunculate oak (Quercus robur) in the northeast of its distribution area. Entomol. Rev. 101, 437–449. doi: 10.1134/S0013873821040011
Franjević, M., Drvodelić, D., Kolar, A., Gradečki-Poštenjak, M., and Hrašovec, B. (2018). “Impact of oak lace bug Corythucha arcuata (Heteroptera: Tingidae) on pedunculate oak (Quercus robur) seed quality” in Natural resources green technology & sustainable development. eds. I. R. Redovniković, T. Jakovljević, V. P. Tominac, M. Panić, R. Stojaković, and D. Erdec, et al., 161–165.
Franjević, M., Matošević, D., and Zorić, N. (2023). Further spread of Corythucha arcuata (Hemiptera; Tingidae) in Croatia. SEEFOR 14, 111–115. doi: 10.15177/seefor.23-06
Glavaš, M. (2011). Zastita hrastovih sastojina od pepelnice (Microsphaera alphitoides Griff. et Maubl.). Croat. J. Eng. 32, 205–210.
Glavendekić, M. (2010). Parasitoids and hyperparasitoids of Erannis defoliaria cl. (Lepidoptera, Geometridae) in oak forests. Šumar. List 134, 403–410.
Harapin, M., and Jurc, M. (2000). A study of important entomofauna in oak forests of Slovenia. Zbornik Gozdarstva in Lesarstva 61, 75–93.
Hariot, M. P. (1907). Note sur un Oidium du Chene. Bulletin de La Societe Mycologique de France 23, 157–159.
Ivashov, A. V., Boyko, G. E., and Simchuk, A. P. (2002). The role of host plant phenology in the development of the oak leafroller moth, Tortrix viridana L (Lepidoptera: Tortricidae). Forest Ecol. Manag. 157, 7–14. doi: 10.1016/S0378-1127(00)00652-6
Jankowiak, R., Stępniewska, H., Bilański, P., and Taerum, S. J. (2022). Fungi as potential factors limiting natural regeneration of pedunculate oak (Quercus robur) in mixed-species forest stands in Poland. Plant Pathol. 71, 805–817. doi: 10.1111/ppa.13529
Karadžić, D. (2010). Šumska fitopatologija. Beograd: Univerzitet u Beogradu-Šumarski fakultet, Univerzitet u Banjoj Luci-Šumarski fakultet.
Karadžić, D., and Milijašević, T. (2005). The most frequent powdery mildews on forest woody species and their impact. Bullet. Facult. Forest. 91, 9–29. doi: 10.2298/GSF0591009K
Kebert, M., Kostić, S., Zlatković, M., Stojnic, S., Čapelja, E., Zorić, M., et al. (2022). Ectomycorrhizal Fungi modulate biochemical response against powdery mildew disease in Quercus robur L. Forests 13:1491. doi: 10.3390/f13091491
Kern, A., Marjanović, H., Csóka, G., Móricz, N., Pernek, M., Hirka, A., et al. (2021). Detecting the oak lace bug infestation in oak forests using MODIS and meteorological data. Agric. For. Meteorol. 306:108436. doi: 10.1016/j.agrformet.2021.108436
Lonsdale, D. (2015). Review of oak mildew, with particular reference to mature and veteran trees in Britain. Arboric. J. 37, 61–84. doi: 10.1080/03071375.2015.1039839
Marçais, B., and Desprez-Loustau, M. L. (2014). European oak powdery mildew: impact on trees, effects of environmental factors, and potential effects of climate change. Ann. For. Sci. 71, 633–642. doi: 10.1007/s13595-012-0252-x
Margaletić, J., Jurjević, V., Glavas, M., Hrašovec, B., and Dimić, D. (2007). The Analises extermination of gypsy moth (Lymantria dispar L.) in the 2005 year of Croatian state forests. Sumar. List 11–12, 539–548.
Marković, Č. (2022). Survey of Cynipid gall wasps (Hymenoptera, Cynipidae) in Serbia. J. Entomol. Res. Soc. 24, 177–193. doi: 10.51963/jers.v24i2.2213
Marković, Č., and Dobrosavljević, J. (2023). Review of Scolytinae (Coleoptera, Curculionidae) of Serbia. J. Entomol. Res. Soc. 25, 545–561. doi: 10.51963/jers.v25i3.2452
Marković, Č., Dobrosavljević, J., and Milanović, S. (2021a). Factors influencing the oak lace bug (Hemiptera: Tingidae) behavior on oaks: feeding preference does not mean better performance? J. Econ. Entomol. 114, 2051–2059. doi: 10.1093/jee/toab148
Marković, Č., Dobrosavljević, J., Vujičić, P., and Cebeci, H. H. (2021b). Impact of regeneration by shelterwood cutting on the pedunculate oak (Quercus robur) leaf mining insect community. Biologia 76, 1197–1203. doi: 10.2478/s11756-020-00631-7
Marković, Č., and Stojanović, A. (2011). Phloemophagous and xylophagous insects, their parasitoids, predators and inquilines in the branches of the most important oak species in Serbia. Biologia 66, 509–517. doi: 10.2478/s11756-011-0049-7
Marović, R., Marović, M., Janjčić, G., and Lazarev, V. (1998). The gypsy moth outbreaks in Serbia. Acta Entomol. Serbica, 7–12.
Mieslerová, B., Cook, R. T. A., Wheater, C. P., and Lebeda, A. (2022). Ecology of powdery mildews–influence of abiotic factors on their development and epidemiology. Crit. Rev. Plant Sci. 41, 365–390. doi: 10.1080/07352689.2022.2138044
Mihajlović, L., and Glavendekić, M. (2006). The most important entomological problems in suburban oak forest in Serbia. Šumarstvo 3, 77–98.
Milanović, S., Milenković, I., Dobrosavljević, J., Popović, M., Solla, A., Tomšovský, M., et al. (2020b). Growth rates of Lymantria dispar larvae and Quercus robur seedlings at elevated CO2 concentration and Phytophthora plurivora infection. Forests 11:1059. doi: 10.3390/f11101059
Milanović, S., Miletić, Z., Marković, Č., Jovanović, D. Š., Trailović, Z., Jankovský, L., et al. (2022). Suitability of Turkey oak, European beech, and hornbeam to gypsy moth feeding. Forests 13:1006. doi: 10.3390/f13071006
Milanović, S., Mladenović, K., Stojnić, B., Solla, A., Milenković, I., Uremović, V., et al. (2021). Relationships between the pathogen Erysiphe alphitoides, the phytophagous mite Schizotetranychus garmani (Acari: Tetranychidae) and the predatory mite Euseius finlandicus (Acari: Phytoseiidae) in oak. Insects 12:981. doi: 10.3390/insects12110981
Milanović, S. D., Popović, M. M., Dobrosavljević, J. N., Kostić, I. M., and Lazarević, J. M. (2020a). Desperate times call for desperate measures: short-term use of the common ash tree by gypsy moth larvae (Lepidoptera: Erebidae) under density and starvation stress. Arch. Biol. Sci. 72, 63–69. doi: 10.2298/ABS191106067M
Mladenović, K., Stojnić, B., Milanović, S., Milenković, I., and Radulović, Z. (2021). Predatory mites and spider mites (Acari: Phytoseiidae and Tetranychidae) on oak trees in Serbia. Acta Zool. Bulg. 73, 179–185.
Mölder, A., Sennhenn-Reulen, H., Fischer, C., Rumpf, H., Schönfelder, E., Stockmann, J., et al. (2019). Success factors for high-quality oak forest (Quercus robur, Q. petraea) regeneration. For. Ecosyst. 6, 1–17. doi: 10.1186/s40663-019-0206-y
Nagy, D., Magura, T., Mizser, S., Debnár, Z., and Tóthmérész, B. (2016). Recovery of surface-dwelling assemblages (Coleoptera: Carabidae, Staphylinidae) during clear-cut originated reforestation with native tree species. Period. Biol. 118, 195–203. doi: 10.18054/pb.2016.118.3.3927
Nikolic, N., Pilipovic, A., Drekic, M., Kojic, D., Poljakovic-Pajnik, L., Orlovic, S., et al. (2019). Physiological responses of Pedunculate oak (Quercus robur L.) to Corythucha arcuata (Say, 1832) attack. Arch. Biol. Sci. 71, 167–176. doi: 10.2298/ABS180927058N
Pajnik, L. P., Drekić, M., Vasić, V., Denić, I., and Fišgar, D. (2017). Testing the efficiency of preparation of Fastac Forst for the protection of logs of common oak from abroad Platypus cylindrus fab. (Coleoptera: Curculionidae). Šumarstvo 3–4, 233–240.
Pap, P., Bobinac, M., and Andrasev, S. (2013). Height growth characteristics of one-year-old pedunculate oaks on regeneration areas with and without fungicide protection against oak powdery mildew (Microsphaera alphitoides Griff. Et Maubl.). Bullet. Facult. Forest. 108, 169–190. doi: 10.2298/GSF130414004P
Pap, P., Drekić, M., Poljaković-Pajnik, L., Marković, M., and Vasić, V. (2014a). The most important insect pests in forest ecosystems of vojvodina and their suppression during the period 2004-2013. Silva Balcanica 15, 68–80.
Pap, P., Drekić, M., Poljaković-Pajnik, L., Marković, M., and Vasić, V. (2015). Forest health monitoring in Vojvodina in 2015. Poplar 188, 117–133.
Pap, P., Ranković, B., and Maširević, S. (2012). Significance and need of powdery mildew control (Microsphaera alphitoides Griff. Et Maubl.) in the process of regeneration of the pedunculate oak (Quercus robur L.) stands in the Ravni Srem area. Period. Biol. 114, 91–102.
Pap, P., Stojnić, S., Nikolić, N., Orlović, S., Marković, M., Vasić, V., et al. (2014b). Impact of Erysiphe alphitoides (Griffon & Maubl.) U. Braun & S. Takam. On leaf physiological parameters in pedunculate oak (Quercus robur L.) saplings. Balt. For. 20, 2–9.
Paulin, M. J., Eötvös, C. B., Zabransky, P., Csóka, G., and Schebeck, M. (2023). Cold tolerance of the invasive oak lace bug, Corythucha arcuata. Agric. For. Entomol. 25, 612–621. doi: 10.1111/afe.12585
Paulin, M., Hirka, A., Eötvös, C. B., Gáspár, C., Fürjes-Mikó, Á., and Csóka, G. (2020). Known and predicted impacts of the invasive oak lace bug (Corythucha arcuata) in European oak ecosystems-A review. Folia Oecol. 47, 131–139. doi: 10.2478/foecol-2020-0015
Pernek, M., Pilas, I., Vrbek, B., Benko, M., Hrasovec, B., and Milkovic, J. (2008). Forecasting the impact of the gypsy moth on lowland hardwood forests by analyzing the cyclical pattern of population and climate data series. For. Ecol. Manag. 255, 1740–1748. doi: 10.1016/j.foreco.2007.11.031
Pilipović, A., Gavranović Markić, A., Orlović, S., Kesić, L., Milović, M., Kovačević, B., et al. (2022). Diversity of ectomycorrhizal Fungi in young Pedunculate oak stand from Morović, Serbia. South-East European For. 13, 19–25. doi: 10.15177/seefor.22-02
Puchałka, R., Koprowski, M., Gričar, J., and Przybylak, R. (2017). Does tree-ring formation follow leaf phenology in Pedunculate oak (Quercus robur L.)? Eur. J. For. Res. 136, 259–268. doi: 10.1007/s10342-017-1026-7
Rađević, V., Pap, P., and Vasić, V. (2020). Management of the common oak forests in Ravni Srem: yesterday, today, tomorrow. Poplar 41–52, 41–52. doi: 10.5937/topola2006041R
Rumiantsev, M., Lukyanets, V., Musienko, S., Mostepanyuk, A., and Obolonyk, I. (2018). Main problems in natural seed regeneration of pedunculate oak (Quercus robur L.) stands in Ukraine. For. Stud. 69, 7–23. doi: 10.2478/fsmu-2018-0008
Simov, N., Grozeva, S., and Langourov, M. M. G., Mirchev, P., and Georgiev, G. (2018). Rapid expansion of the oak lace bug Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae) in Bulgaria. Hist. Nat. Bulg. 27, 51–55.
Stancă-Moise, C., Moise, G., Rotaru, M., Vonica, G., and Sanislau, D. (2023). Study on the ecology, biology and ethology of the invasive species Corythucha arcuata Say, 1832 (Heteroptera: Tingidae), a danger to Quercus spp. in the climatic conditions of the City of Sibiu, Romania. Forests 14:1278. doi: 10.3390/f14061278
Tikkanen, O. P., and Julkunen-Tiitto, R. (2003). Phenological variation as protection against defoliating insects: the case of Quercus robur and Operophtera brumata. Oecologia 136, 244–251. doi: 10.1007/s00442-003-1267-7
Tomescu, R., and Netoiu, C. (2008). “Control of the Broak leave’s main defoliators in Romania in 2005,” in IUFRO working party 7.03.10 proceedings of the workshop 2006. ed. U. Hoyer-Tomiczek (Gmunden, Vienna, Austria: BFW), 263–270.
Tomescu, R., Olenici, N., Netoiu, C., Bǎlǎcenoiu, F., and Buzatu, A. (2018). Invasion of the oak lace bug Corythucha arcuata (say.) in Romania: A first extended reporting. Ann. For. Res. 61, 161–170. doi: 10.15287/afr.2018.1187
Valdés-Correcher, E., Maarten, C., Laura, D. G., Alex, S., and Yannick, S. (2023). Impact of early insect herbivory on the invasive oak lace bug (Corythucha arcuata Say, 1832) in different oak species. Arthropod Plant Interact. 17, 363–371. doi: 10.1007/s11829-023-09967-8
Wrzesińska, D. (2017). Insects mining leaves of English oak Quercus robur L. in Bydgoszcz and its vicinit. For. Res. Pap. 78, 337–345. doi: 10.1515/frp-2017-0038
Zargaran, M. R., Erbilgin, N., and Ghosta, Y. (2012). Changes in oak gall wasps species diversity (Hymenoptera: Cynipidae) in relation to the presence of oak powdery mildew (Erysiphe alphitoides). Zool. Stud. 51, 175–184.
Keywords: foliofagous insects, chemical control, Corythucha arcuata , defoliators, Erysiphe alphitoides , Quercus robur , height growth
Citation: Marković &, Kanjevac B, Perišić U and Dobrosavljević J (2024) The effect of the oak powdery mildew, oak lace bug, and other foliofagous insects on the growth of young pedunculate oak trees. Front. For. Glob. Change. 6:1297560. doi: 10.3389/ffgc.2023.1297560
Edited by:
Milica Zlatkovic, University of Novi Sad, SerbiaReviewed by:
Flavius Balacenoiu, National Institute for research and Development in Forestry Marin Dracea (INCDS), RomaniaJelena Kranjec Orlović, University of Zagreb, Croatia
Mihajlo Risteski, Saints Cyril and Methodius University of Skopje, North Macedonia
Copyright © 2024 Marković, Kanjevac, Perišić and Dobrosavljević. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jovan Dobrosavljević, jovan.dobrosavljevic@sfb.bg.ac.rs
 Čedomir Marković1
Čedomir Marković1  Branko Kanjevac
Branko Kanjevac Jovan Dobrosavljević
Jovan Dobrosavljević