Immunometabolic Analysis of Mobiluncus mulieris and Eggerthella sp. Reveals Novel Insights Into Their Pathogenic Contributions to the Hallmarks of Bacterial Vaginosis
- 1Department of Obstetrics and Gynecology, College of Medicine-Phoenix, University of Arizona, Phoenix, AZ, United States
- 2Department of Biology and Biochemistry, University of Bath, Bath, United Kingdom
- 3Department of Basic Medical Sciences, College of Medicine-Phoenix, University of Arizona, Phoenix, AZ, United States
The cervicovaginal microbiome plays an important role in protecting women from dysbiosis and infection caused by pathogenic microorganisms. In healthy reproductive-age women the cervicovaginal microbiome is predominantly colonized by protective Lactobacillus spp. The loss of these protective bacteria leads to colonization of the cervicovaginal microenvironment by pathogenic microorganisms resulting in dysbiosis and bacterial vaginosis (BV). Mobiluncus mulieris and Eggerthella sp. are two of the many anaerobes that can contribute to BV, a condition associated with multiple adverse obstetric and gynecological outcomes. M. mulieris has been linked to high Nugent scores (relating to BV morphotypes) and preterm birth (PTB), whilst some bacterial members of the Eggerthellaceae family are highly prevalent in BV, and identified in ~85-95% of cases. The functional impact of M. mulieris and Eggerthella sp. in BV is still poorly understood. To determine the individual immunometabolic contributions of Eggerthella sp. and M. mulieris within the cervicovaginal microenvironment, we utilized our well-characterized human three-dimensional (3-D) cervical epithelial cell model in combination with multiplex immunoassays and global untargeted metabolomics approaches to identify key immune mediators and metabolites related to M. mulieris and Eggerthella sp. infections. We found that infection with M. mulieris significantly elevated multiple proinflammatory markers (IL-6, IL-8, TNF-α and MCP-1) and altered metabolites related to energy metabolism (nicotinamide and succinate) and oxidative stress (cysteinylglycine, cysteinylglycine disulfide and 2-hydroxygluatrate). Eggerthella sp. infection significantly elevated multiple sphingolipids and glycerolipids related to epithelial barrier function, and biogenic amines (putrescine and cadaverine) associated with elevated vaginal pH, vaginal amine odor and vaginal discharge. Our study elucidated that M. mulieris elevated multiple proinflammatory markers relating to PTB and STI acquisition, as well as altered energy metabolism and oxidative stress, whilst Eggerthella sp. upregulated multiple biogenic amines associated with the clinical diagnostic criteria of BV. Future studies are needed to evaluate how these bacteria interact with other BV-associated bacteria within the cervicovaginal microenvironment.
Introduction
In healthy reproductive age women, the cervicovaginal microbiome is generally dominated by Lactobacillus spp. These beneficial bacteria acidify the cervicovaginal microenvironment via lactic acid production, which contributes to protection against infections by pathogenic and opportunistic microorganisms (O’hanlon et al., 2011). The depletion of Lactobacillus spp. leads to the colonization of the lower female reproductive tract (FRT) by a diverse consortium of facultative and obligate anaerobic bacteria, a disorder is referred to as bacterial vaginosis (BV) (Workowski and Bolan, 2015; Muzny et al., 2020). Importantly, BV is associated with a range of adverse gynecologic and obstetric outcomes including an increased risk of sexually transmitted infections (STI) and preterm birth (PTB). Microbiologically, BV is characterized by the presence of a polymicrobial biofilm covering the surface of cervicovaginal epithelium (Muzny et al., 2019). Mobiluncus mulieris and Eggerthella sp. are two of the many anaerobes that may contribute to the biofilm formation, yet their mechanistic contributions to BV and related adverse gynecologic and obstetric outcomes are still poorly understood (Danielsson et al., 2011; Machado and Cerca, 2015).
Mobiluncus spp. are motile, curved rod-shaped bacteria that are isolated from vaginal secretions from women with BV and are associated with high Nugent scores (a method to diagnose BV) (Sprott et al., 1983; Roberts et al., 1985; Hallén et al., 1987; Teo et al., 1987; Vetere et al., 1987; Moi et al., 1991; Gatti, 2000; Srinivasan and Fredricks, 2008). In addition, M. mulieris has been isolated from extragenital sites, such as breast and umbilical abscesses (Glupczynski et al., 1984). In previous epidemiological studies, M. mulieris have been also linked to PTB (Holst et al., 1994; Hillier et al., 1995; Meis et al., 1995). Genital inflammation has been implicated in PTB and M. mulieris has been hypothesized as a microbial driver in such inflammatory states (Dude et al., 2020). The flagella of M. mulieris has been previously demonstrated to stimulate Toll-like receptor 5 (TLR5) activation, which links to the elevation of key inflammatory markers (IL-6, IL-8, and TNF-α) and PTB (Anahtar et al., 2015; Onderdonk et al., 2016; Dude et al., 2020; Dela Cruz et al., 2021). In addition, M. mulieris has been shown to exert sialidase activity (Culhane et al., 2006). Notably, this bacterial enzyme cleaves sialic acid from highly glycosylated proteins present in the cervical mucus plug and its activity is associated with BV (Smayevsky et al., 2001), PTB and chorioamnionitis (Critchfield et al., 2013; Racicot et al., 2013; Smith-Dupont et al., 2017).
Some bacterial members of the Eggerthellaceae family are highly prevalent in BV, identified in ~85-95% of cases (Fredricks et al., 2005; Fredricks et al., 2007; Srinivasan et al., 2012; Shipitsyna et al., 2013). Interestingly, one member of the Eggerthellaceae family have also been linked to all four of the Amsel criteria (vaginal pH, vaginal odor, vaginal discharge and the presence of clue cells) used to diagnose BV in clinical settings (Srinivasan et al., 2015). Eggerthella spp. [previously classified as Eubacterium (Kageyama et al., 1999)] are non-motile anaerobic coccobacilli that are part of the healthy human gut microbiome (Finegold et al., 1983; Schwiertz et al., 2000). The taxonomy of the Eggerthellaceae family requires further investigation to classify them into their appropriate genus and species. However, Eggerthella spp. can also cause bacteremia and sepsis with high mortality rates (Lau et al., 2004a; Lau et al., 2004b; Thota et al., 2011; Lee et al., 2012). This suggests that in the FRT, Eggerthella spp. might play a role in the pathophysiological processes that manifest as adverse obstetric and gynecologic outcomes.
To determine the individual immunometabolic contributions of Eggerthella sp. and M. mulieris within the cervical microenvironment, we utilized our well-characterized human three-dimensional (3-D) cervical epithelial cell model that recapitulates several physiologically relevant features of in vivo tissue, including TLR expression, microvilli, intercellular junctional complexes and secretory material. We combined this advanced bioreactor-derived 3-D cell culture model with multiplex immunoassay and global untargeted metabolomics approaches to identify key immune mediators and metabolites related to M. mulieris and Eggerthella sp. infections of the lower FRT. We chose the 3-D cervical model since the cervix is a critical area impacted by cervicovaginal microbiota that, when disrupted, can lead to PTB, increased STI acquisition and other gynecological sequalae associated with BV.
Methods
Human Cervical Epithelial Cell Culture and Generation of the 3-D Cervical Model
Human cervical epithelial cells (A2EN) were generously provided by Dr. Alison Quayle at Louisiana State University Health Sciences Center (Herbst-Kralovetz et al., 2008; Buckner et al., 2011) and were routinely maintained in keratinocyte serum-free media (KSFM) (Fisher Scientific) supplemented with epidermal growth factor (5 ng/ml), bovine pituitary extract (50 µg/ml), CaCl2 (Gibco) and primocin (100 µg/ml; In vivoGen) at 37°C in a 5% carbon dioxide (CO2) humidified atmosphere. Short tandem repeat DNA profiling confirmed that cells were not contaminated with other cell lines found in available databases. For downstream experiments, we used cervical epithelial cells (passage ~50–60) cultured as monolayers or 3-D cervical cell models. Monolayer cultures were seeded at ~2 × 105 cells/ml into tissue culture-treated 24-well plates. Prior to seeding, cells were enumerated by trypan blue exclusion. The 3-D cervical cell models were generated as previously described (Radtke and Herbst-Kralovetz, 2012; Radtke et al., 2012; Jackson et al., 2020). Briefly, cervical epithelial cell monolayers were trypsinized and counted using a Countess automated cell counter (Invitrogen). The single cell suspension (~1 × 107) was combined with 300 mg of hydrated Cytodex-3 collagen-coated dextran microcarrier beads (Sigma-Aldrich) suspended in pre-warmed KFSM-primocin medium. The mixture was transferred to a rotating-wall vessel (RWV) bioreactor (Synthecon). Bioreactors were incubated at 37°C for 28-days at 20 rpm, with daily medium changes. After 28-days the 3-D cervical cell models were harvested, washed and resuspended in antibiotic-free KFSM medium, enumerated, and distributed into 24-well plates at a density of ~5 x 105 cells/well for downstream experiments.
Bacterial Strains and Growth Conditions
All bacterial strains used in this study were obtained from the Biodefense and Emerging Infections (BEI) Research Repository (NIAID, NIH as a part of the Human Microbiome Project). M. mulieris strain UPII-28I and Eggerthella sp. strain MVA1 were cultured on tryptic soy agar (TSA) (Becton Dickinson) supplemented with 5% defibrinated sheep blood (Quad Five) at 37°C under anaerobic conditions generated using anaerobic environment chambers and AnaeroPacks (Thermo Scientific). Due to large taxonomic restructuring of vaginal species over the last five years we decided to confirm our strains taxonomic classification. Although not much genomic information is available yet for Eggerthella sp. strain MVA1 there is a sequence read SRX655730 in the NCBI Sequence Read Archive. This read in the SRA reports that Eggerthella sp. MVA1 has 86.46% sequence identity with the Eggerthellaceae family and 83.23% identity with the Eggerthella genus using their Sequence Taxonomic Analysis Tool (STAT) (Katz et al., 2021). There is also a 16S rRNA sequence (JX103988) available that has 99% sequence identity with Eggerthella lenta. Future comparative genomic analyses are needed to designate a species for Eggerthella sp. strain MVA1.
Bacterial Infections
M. mulieris UPII-28I and Eggerthella sp. MVA1 were cultured on TSA agar with sheep’s blood for 16-18 hours prior to infection. Bacterial strains were harvested and resuspended in sterile Dulbecco’s phosphate-buffered saline (PBS) and adjusted to an optical density at 600 nm (OD600) for infection assays. The OD600 0.5 reflected the CFU/ml range of 1 x 108 – 1 x 109, likely due to bacterial cell clumping as observed on the SEM. Monolayers were infected with adjusted bacterial suspensions (20 μl of bacterial suspension adjusted to OD600 of 0.05, 0.5 and 5.0 per 1 x 105 cells and incubated for 24 hours under anaerobic conditions at 37°C for use in cytotoxicity assays. The 3-D cervical cell aggregates were infected with adjusted bacterial suspensions (20 μl of bacterial suspension adjusted to OD600 of 0.5 per 1 x 105 cells and incubated under anaerobic conditions at 37°C for 24-hours. In a preliminary experiment the bacterial recovery 24 hours after the infection of the 3-D cervical cell model with both M. mulieris UPII-28I and Eggerthella sp. MVA1 was within 0.5 of a log of the initial infection dose. PBS-treated cells served as mock-infected controls. Culture supernatants were immediately used for cytotoxicity assays or stored at -80°C for downstream immunoproteomic and metabolomic analyses.
Lactate Dehydrogenase Assay (LDH)
Culture supernatants from cervical epithelial monolayer cell infections were used to assess cytotoxicity using the CyQUANT LDH assay (Thermo Fisher Scientific) according to the manufacturer’s protocol. LDH activity was measured by recording absorbance values at 490 nm and 680 nm and the percentage LDH activity was calculated according to the equation: The assay was performed using three independent biological replicates.
Scanning Electron Microscopy
Human 3-D cervical cell models were infected with M. mulieris UPII-28I and Eggerthella sp. MVA1 for four hours under anaerobic conditions at 37°C. Samples were fixed in 2.5% glutaraldehyde (Electron Microscopy Sciences) and prepared for scanning electron microscopy (SEM) as described previously (Hjelm et al., 2010; Mcgowin et al., 2013). Infected 3-D cervical cell aggregates were imaged with a JSM-6300 JEOL scanning electron microscope and IXRF model 500 digital processor (IXRF systems) at the Electron Microscopy Core at Arizona State University. Representative images collected for each bacterium were selected for inclusion in the figure. Pseudo-coloring of the SEM images was performed using Adobe Photoshop CS6 v13.
Multiplex Immunoassays
Cell culture supernatants from 3-D cervical cell models infected with M. mulieris UPII-28I and Eggerthella sp. MVA1 were collected from three independent experiments. The levels of five cytokines: (interleukin (IL)-1α, IL-1β, IL-1RA, IL-6, tumor necrosis factor-α (TNF)-α), seven chemokines: fractalkine, IL-8, interferon γ-induced protein-10 (IP-10), monocyte chemoattractant protein (MCP)-1, MCP-3, macrophage inflammatory protein-1β (MIP-1β), regulation on activation, normal T-cell expressed and secreted (RANTES) and three growth factors: platelet derived growth factor-AA (PDGF-AA), transforming growth factor-α (TGF-α), vascular endothelial growth factor (VEGF) were measured using customized MILLIPLEX® multianalyte profiling (MAP) Human Cytokine/Chemokine Panel 1 array (Millipore) and compared to PBS mock infections. Data was collected using a Bio-Plex® 200 (Bio-Rad) platform and evaluated using Manager (5.0) software (Bio-Rad). A five-parameter logistic regression curve fit was used to determine the concentration. All samples were analyzed in biological triplicate, each containing two technical replicates.
Untargeted Metabolomics Analysis
Cell culture supernatants from 3-D cervical cell models infected with M. mulieris UPII-28I and Eggerthella sp. MVA1 from three independent experiments were sent to Metabolon Inc. (Durham, NC) for untargeted global metabolomics analysis. Metabolites were resolved using ultra-performance liquid chromatography with mass spectrometry (UPLC-MS) as described previously (Ilhan et al., 2020; Salliss et al., 2021). The sample extracts were dried then reconstituted in solvents compatible to four different methods. Sample aliquots were analyzed using: acidic positive ion conditions that were chromatographically optimized for more hydrophilic or hydrophobic compounds, basic negative ion optimized conditions and negative ionization conditions. The MS analysis used dynamic exclusion with a scan range covering 70-1000 m/z. The Laboratory Information Management System (LIMS) was used for data extraction and peak-identification, QC and compound identification.
Statistical Analysis
All assays and infections were performed as at least three biological replicates. Statistical differences between the mean protein concentrations among groups were determined by one-way ANOVA with Bonferroni post-hoc test using Prism v9.1.1 software (GraphPad). ClustVis (Metsalu and Vilo, 2015) was used to perform hierarchical clustering analysis (HCA) on the Bio-Plex data (ln-transformed and Pareto scaled, Euclidean distance measures and average linkage clustering). Metabolomics data analyses, including HCA, Spearman’s correlation analysis, principal component analysis (PCA) and metabolite enrichment pathway analysis, were performed with MetaboAnalyst 5.0 (Pang et al., 2021). Prior to analysis the metabolomics data was log-transformed, and Pareto scaled. Relative abundance is the normalized values from the area under the curve of the metabolite peaks collected that are rescaled to set the median equal to 1, before inputting any missing values as the minimum. To determine the significance between the mean relative abundances of metabolites among groups (infection vs. PBS control), two-tailed paired Student’s t-tests was performed using the rstatix R package. To correct for multiple comparisons, p-values were adjusted using false discovery rate (FDR) and q-values were reported. p-values below 0.05 were considered significant.
Results
Eggerthella sp. and Mobiluncus mulieris Do Not Induce Significant Cytotoxicity in Colonized 3-D Cervical Epithelial Cell Models
First, we assessed whether Eggerthella sp. and M. mulieris infections induced cytotoxicity in cervical epithelial cell monolayers at three doses which corresponded with the final OD600 of 0.1, 0.01 and 0.001 of 1x105 cervical cells/ml. Using LDH cytotoxicity assays, we found that there was no significant cytotoxicity induced following infection with Eggerthella sp. and M. mulieris at any dose tested (Supplementary Figure 1).
We confirmed colonization of 3-D cervical cell models with Eggerthella sp. and M. mulieris by SEM (Figure 1). Both Eggerthella sp. and M. mulieris formed clusters and interacted simultaneously with multiple cells in some areas. Eggerthella sp. colonized the 3-D cervical cell models in smaller clusters and longer chains (Figures 1A, B). M. mulieris exhibited flagella-like structures which appeared to interact with other bacterial cells (Figure 1C) and epithelial cell surfaces (Figure 1D).
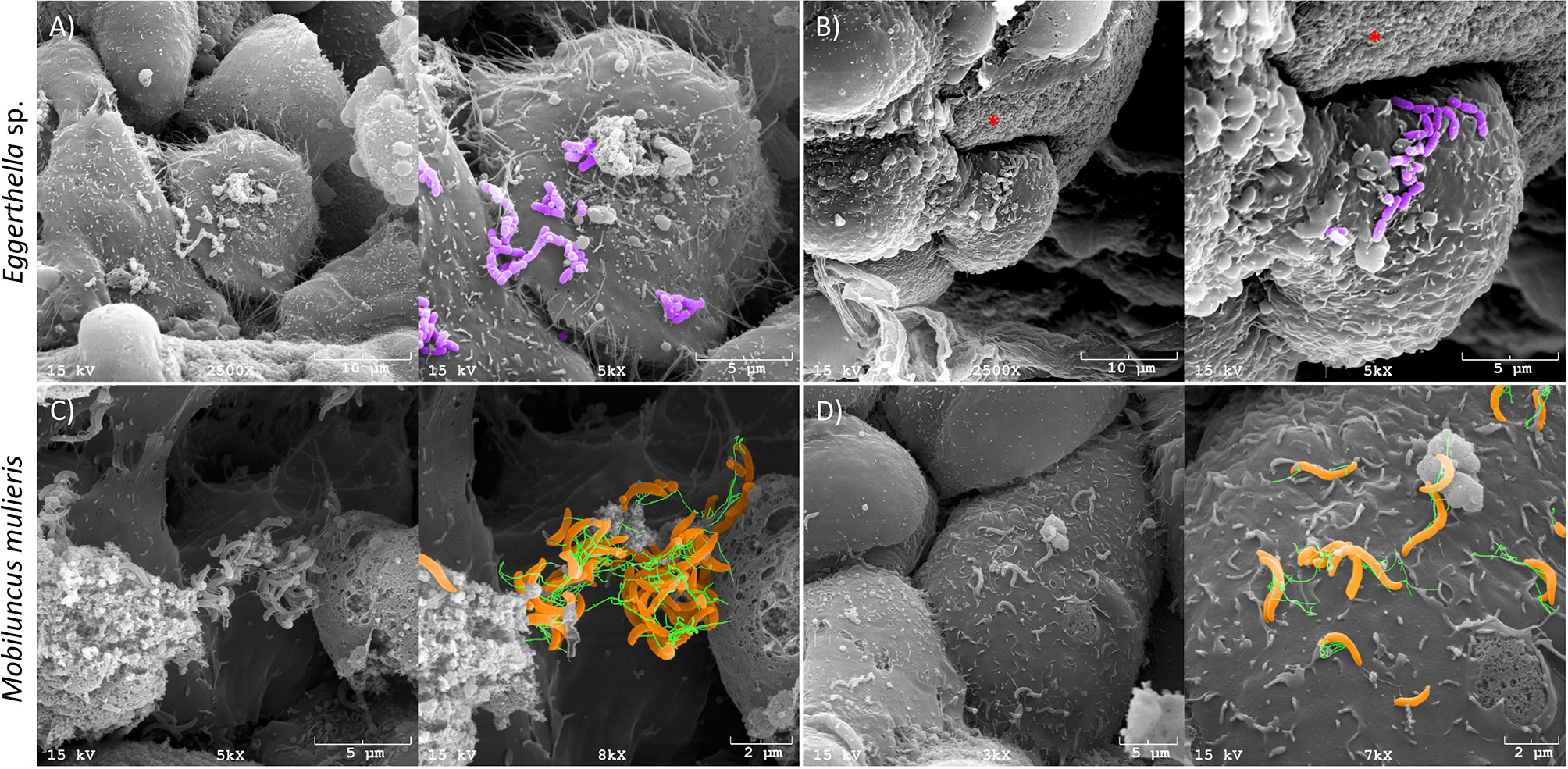
Figure 1 Eggerthella sp. and M. mulieris colonize 3-D human cervical epithelial cell models. Pseudo-colored scanning electron microscopy (SEM) images of (A) and (B), Eggerthella sp. MVA1 and (C, D), M. mulieris UPII-28I showing colonization of 3-D cervical epithelial cells. Eggerthella sp. MVA1 exhibit a coccobacilli morphology and colonized 3-D cervical cells in small clusters or short chains. M. mulieris UPII-28I cells exhibit a curved rod-shaped morphology and were pseudo-colored orange with flagella-like structures pseudo-colored green. The * indicates the collagen-coated microcarrier beads used to generate the 3-D cervical epithelial cell models.
Infection of 3-D Cervical Aggregates With M. mulieris Upregulated Levels of Several Key Proinflammatory Cytokines and Chemokines, Whereas Infection With Eggerthella sp. Elevated IL-1α Secretion
To investigate the host immune response to M. mulieris and Eggerthella sp., we infected 3-D cervical cell models with each bacterium for 24 hours and measured levels of secreted cytokines (IL-1α, IL-1β, IL-1RA, IL-6, TNF-α), chemokines (fractalkine, IL-8, IP-10, MCP-1, MCP-3, MIP-1β, RANTES) and growth factors (PDGF-AA, TGF-α, VEGF). Data from the infectious conditions were compared to PBS mock-infected controls.
We performed hierarchical clustering analysis (HCA) to visualize patterns of immune mediator expression by 3-D cervical cell models in response to bacterial infection (Figure 2A). HCA demonstrated distinct immune mediator profiles of M. mulieris and Eggerthella sp. as each condition clustered separately from the PBS mock-infected controls. Using the multiplex assays, we found that infection of 3-D cervical cell models with M. mulieris significantly upregulated expression of IL-6 (p<0.0001), IL-8 (p<0.0001), MCP-1 (p<0.01) and TNF-α (p<0.01) whereas infection with Eggerthella sp. significantly upregulated only IL-1α (p=0.01) (Figure 2B and Supplementary Figure 2). IL-6, IL-8, TNF-α and IL-1α are all proteins linked to increased genital inflammation (Hannun and Obeid, 2018; Łaniewski et al., 2018). This data indicated that M. mulieris promoted a proinflammatory response in 3-D cervical cell models to a greater extent than Eggerthella sp.
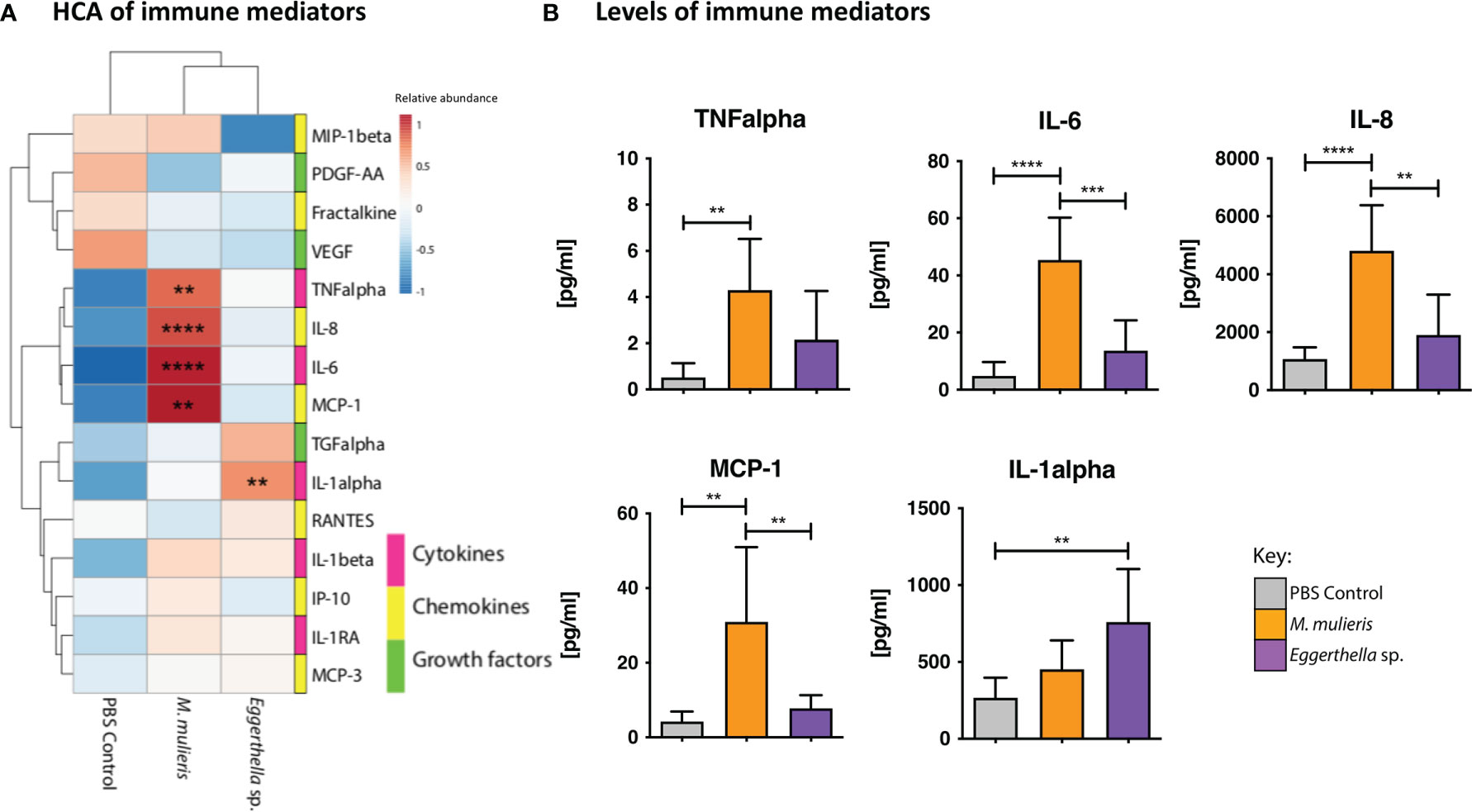
Figure 2 M. mulieris significantly elevated production of inflammatory cytokines and chemokines compared to Eggerthella sp. and PBS mock-infected controls in 3-D cervical aggregates. (A) Hierarchical clustering analysis (HCA) of cytokine, chemokine, and growth factor profiles secreted by 3-D cervical cells in response to infection with Eggerthella sp. MVA1 or M. mulieris UPII-28I. The data was log-transformed and Pareto-scaled prior to clustering. HCA was performed using Euclidean distance measures and average linkage clustering algorithms. (B) Bio-Plex analysis of cytokines, chemokines and growth factors secreted by 3-D human cervical cells infected with M. mulieris UPII-28I and Eggerthella sp. MVA1 for 24h in anaerobic conditions. TNF-α, IL-6, IL-8 and MCP-1 were all significantly elevated by M. mulieris UPII-28I, whilst Eggerthella sp. MVA1 significantly increased expression of IL-1α. Statistical significance was determined by one-way ANOVA and Bonferroni post-hoc multiple comparisons. **, p<0.01; ***, p<0.001; ****, p<0.0001.
Eggerthella sp. and M. mulieris Infections Distinctly Altered Extracellular Metabolomes Corresponding to Amino Acid and Lipid Superpathways in 3-D Cervical Epithelial Cell Models
To discern the effect of Eggerthella sp. and M. mulieris infections on the cervicovaginal extracellular metabolome, we performed untargeted global metabolomics analysis using supernatants collected from 3-D model experiments. The metabolomics analysis identified 314 known metabolites. To compare global metabolic profiles of Eggerthella sp. and M. mulieris, principal component analysis (PCA) and Spearman’s correlation analysis (Figure 3) were employed. Biological replicates from each bacterial infection and PBS mock-infected controls clustered together and showed distinct separation of each condition by PCA (Figure 3A). Principal component 1 (PC1) explained 42% of variance and was significantly different (p<0.001) between Eggerthella sp. and mock-infected controls; principal component 2 (PC2) explained 21.2% of the variance scores and contributed to separation of M. mulieris and Eggerthella sp. from the mock-infected controls (p<0.05). Spearman’s correlation analysis showed each bacterial infection and the PBS mock-infected controls clustered distinctly from one another with each of the biological replicates grouped together (Figure 3B), therefore showing good replicability, and supporting the PCA analysis.
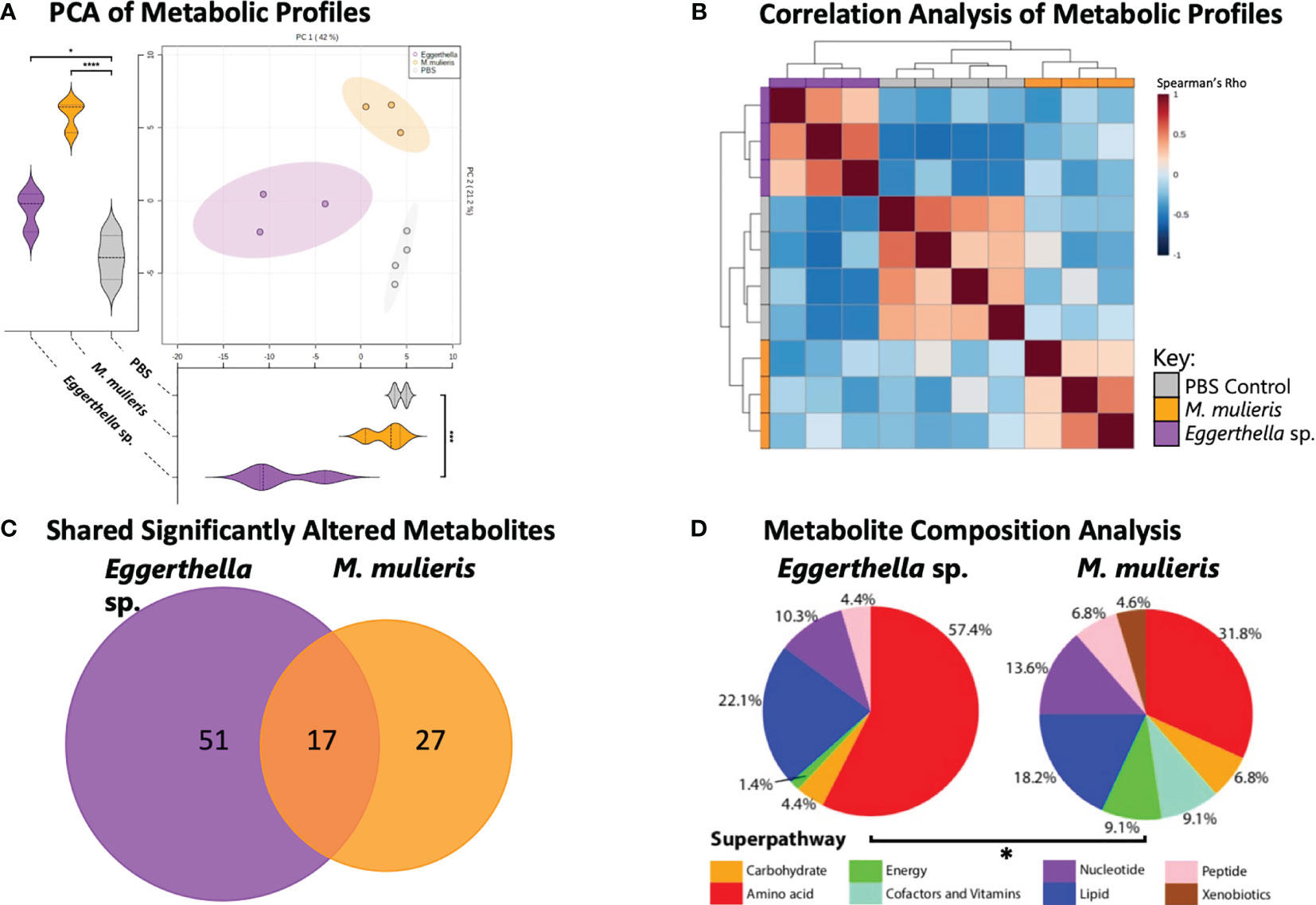
Figure 3 M. mulieris and Eggerthella sp. infections of 3-D cervical cell models resulted in distinct metabolic profiles. (A) Principal component analysis (PCA) shows distinct clustering between each bacterial metabolic profiles and the PBS mock-infected controls. PC1 and PC2 score significance was determined using one-way ANOVA with Bonferroni post-hoc tests. *, p<0.05; ***, p<0.001; ****, p<0.0001. (B) Spearman’s correlation heatmap of metabolic profiles demonstrating clustering of biological replicates for each infection. (C) Venn diagram indicating the unique or overlapping metabolites that were significantly altered (p<0.05) between the two bacterial infections. The significant differences in metabolite abundances among infections were determined using Student’s t-tests with Welch’s correction and compared to PBS mock-infected controls. (D) Pie charts showing the percentage of significantly (p<0.05) altered metabolites grouped by superpathway compared to PBS mock-infected controls (total number of significantly changed metabolites for Eggerthella sp. MVA1 and M. mulieris UPII-28I were 68 and 44 respectively). The significant difference between composition of superpathways was determined with chi-squared (χ2) test (*, p<0.05).
Overall, infection with Eggerthella sp. and M. mulieris significantly (p<0.05) altered the abundance of 68 and 44 metabolites, respectively, compared to mock-infected controls (Supplementary Figure 3). Of these differentially abundant metabolites, Eggerthella sp. and M. mulieris shared 17 significantly altered metabolites (Figure 3C). Next, we grouped significantly altered metabolites by superpathway and compared superpathway profiles between the two bacterial infections. Metabolites representing the amino acid superpathway (57.4% and 31.8% respectively) and the lipid superpathway (22.1% and 18.2% respectively) were profoundly influenced by infection with Eggerthella sp. and M. mulieris (Figure 3D). The overall composition of the superpathways between Eggerthella sp. and M. mulieris was significantly different (p=0.0147).
Next, we conducted metabolic pathway enrichment analysis on the metabolomics data sets to identify metabolic pathways significantly enriched by each bacterial infection (Figure 4). Eggerthella sp. infection significantly (p<0.05) enriched 23 subpathways, mostly associated with the amino acid superpathway (Figure 4A) while M. mulieris infection significantly enriched 24 subpathways and the most significant were from the lipid superpathway (Figure 4B). We also compared and contrasted these subpathways between Eggerthella sp. and M. mulieris (Figures 4C, D). Following comparisons of amino acid and lipid subpathways, we observed that Eggerthella sp. enriched a vast number of amino acid subpathways, twice that of M. mulieris. The majority of subpathways enriched by M. mulieris were also enriched by Eggerthella sp. Conversely, we noted that M. mulieris enriched twice the number of lipid subpathways than Eggerthella sp., with only sphingolipid metabolism being unique to Eggerthella sp. infection.
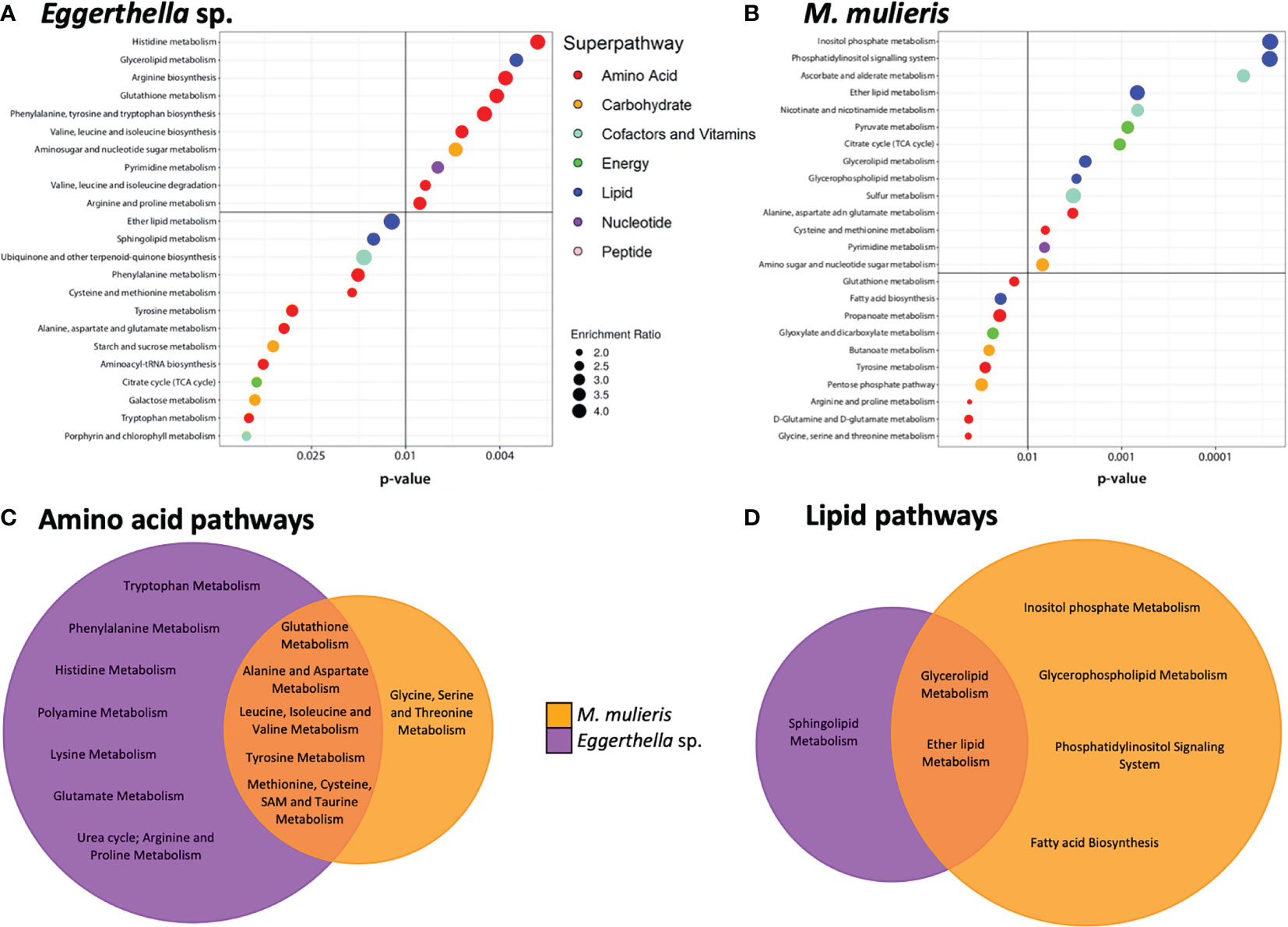
Figure 4 Eggerthella sp. primarily altered amino acid subpathways whilst M. mulieris significantly altered lipid-related subpathways. Metabolic pathway enrichment analysis for (A) Eggerthella sp. MVA1 and (B) M. mulieris UPII-28I infections of 3-D cervical cell models. All subpathways shown were significantly enriched (p<0.05) using metabolite set enrichment analysis (MSEA). Colored circles next to the subpathways indicate which superpathway each subpathway belongs to. Venn diagrams comparing the significantly altered (p<0.05) (C) amino acid and (D) lipid subpathways by Eggerthella sp. MVA1 and M. mulieris UPII-28I infections.
Eggerthella sp. Infection Significantly Altered Levels of Sphingolipids and M. mulieris Infections Significantly Altered Levels of Long-Chain Fatty Acids
Since both M. mulieris and Eggerthella sp. infections significantly modulated lipid metabolic pathways in culture supernatants, we identified the specific lipids with differential abundance (p<0.05) between bacterial infections compared to PBS mock-infected controls. We found 21 significantly altered lipids between both bacterial infections (Figure 5A and Supplementary Figure 4). These lipids can be classified into three categories of metabolism: sphingolipid metabolism, glycerolipid metabolism and inositol phosphate metabolism. Overall, Eggerthella sp. induced differential abundance of more lipids (16) than M. mulieris (7) and both significantly depleted the levels of glycerol (p=0.024 and p=0.0433, respectively) and glycerophosphorylcholine (GPC) p=0.0389 and p=0.00931, respectively) (Figure 5C). Eggerthella sp. infection mainly resulted in accumulation of glycerolipids and sphingolipids in contrast to M. mulieris which predominantly depleted long chain fatty acids; arachidate (p=0.0368), margarate (p=0.000919) and stearate (p=0.00504) (Figure 5B). Interestingly, the sphingolipids that were significantly altered by Eggerthella sp. were also elevated following M. mulieris infections but did not reach significance following infection with the latter species (Figure 5D and Supplementary Figure 4). Sphingolipids are closely linked to epithelial barrier function and inflammation (Hannun and Obeid, 2018; Harrison et al., 2018).
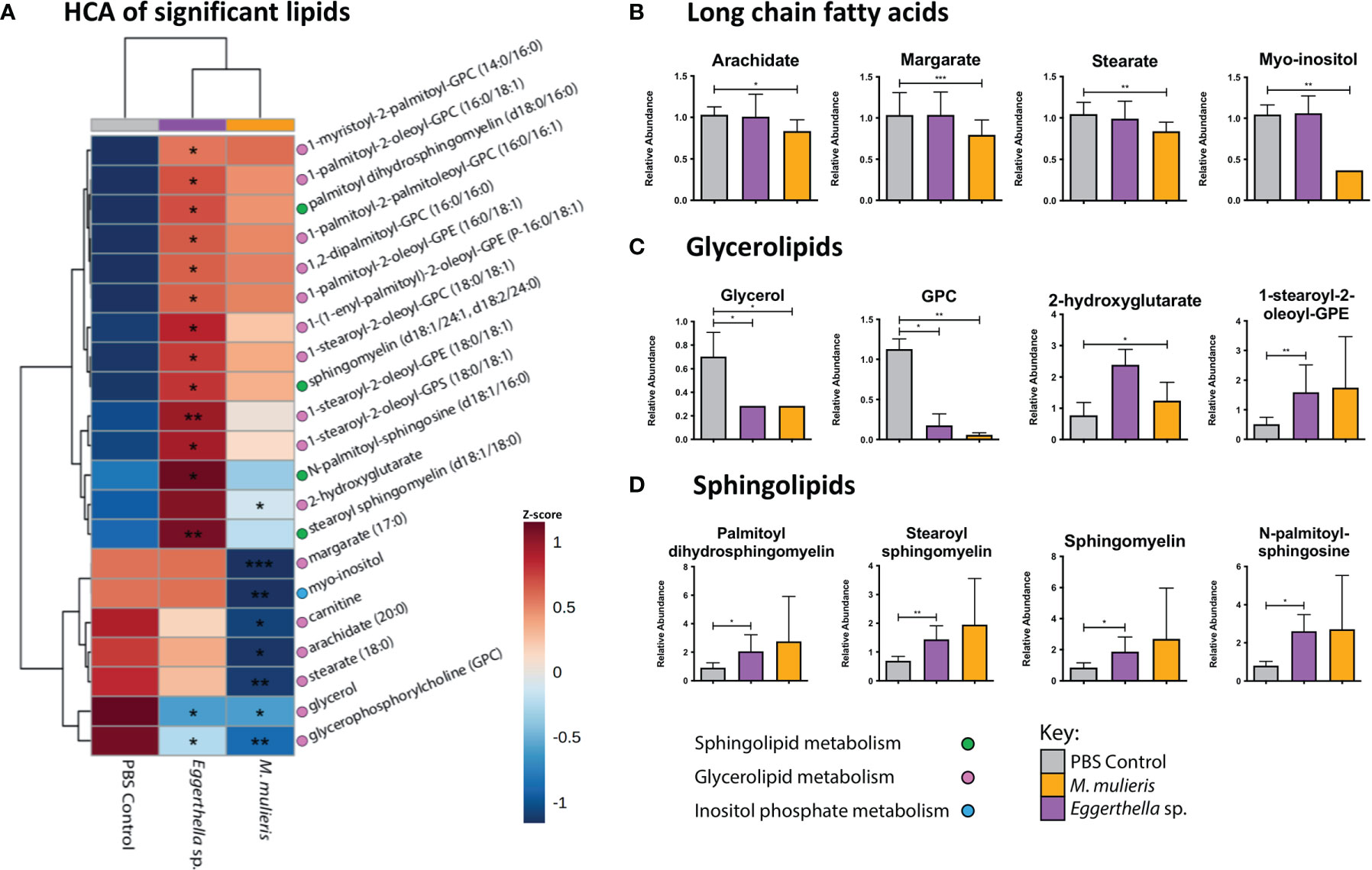
Figure 5 Eggerthella sp. significantly altered the abundance of more lipids than M. mulieris. (A) Hierarchical clustering analysis (HCA) of differentially abundant lipids (p<0.05) determined by Students t-tests with Welch’s correction of Eggerthella sp. MVA1 and M. mulieris UPII-28I infection compared to the PBS mock-infected control. HCA was performed using Euclidean distance measures and average linkage clustering algorithms. Relative abundance graphs of significant lipids classified into long-chain fatty acids (B), glycerolipids (C) and sphingolipids (D). The brackets after the lipids indicate how many carbons and how many double bonds there are in the structure of the lipid. The slash between numbers separates the information about the two hydrocarbon chains of the lipid, whilst the P- prefix indicates a neutral plasmalogen species and the d for sphingomyelins indicates a 1,3 dihydroxy chain. Significant differences between the bacteria and PBS mock-infected controls. *, p<0.05; **, p<0.01; ***, p<0.001.
Eggerthella sp. Infection Significantly Elevated Biogenic Amines and Other Metabolites Associated With BV Symptoms and Diagnosis, Whereas M. mulieris Infection Modulated Metabolites Related to Energy Metabolism and Oxidative Stress
In clinical settings, BV is often diagnosed using the Amsel criteria (Amsel et al., 1983) which are based on the main symptoms of BV (vaginal pH, vaginal odor, vaginal discharge and the presence of clue cells). Thus, we determined whether infection with Eggerthella sp. or M. mulieris induced differential abundance of metabolites related to BV diagnosis in our 3-D human cervical cell models (Figure 6). We also evaluated the metabolites previously identified in cervicovaginal lavages collected from women with BV (Srinivasan et al., 2015). It is well established that biogenic amines are strongly associated with BV (Nelson et al., 2015) and linked to vaginal odor and elevated pH (Srinivasan et al., 2015; Borgogna et al., 2021). Cell culture supernatants from the 3-D cervical cell models infected with Eggerthella sp. significantly accumulated the biogenic amines cadaverine (p=0.0373) and putrescine (p=0.0101) and their precursors citrulline (p=0.00598) and ornithine (p=0.00669), as well as several other BV-related metabolites (Figure 6A, Supplementary Figure 5). In contrast, M. mulieris infections did not result in accumulation of any biogenic amines detected in our samples. M. mulieris infection significantly influenced metabolites related to energy metabolism: nicotinamide (p=0.00429) and succinate (p=0.0335); and oxidative stress: 2-hydroxyglutarate (p=0.0365) and cysteinylglycine (p=0.00103) (Figure 6B and Supplementary Figure 5). Intriguingly, relative abundance of one BV-related metabolite, N-acetylneuraminate (sialic acid), was significantly and differentially altered by both bacteria. Sialic acid was significantly increased by Eggerthella sp. (p=0.0376) and significantly decreased by M. mulieris (p=0.00886), which suggested that both bacteria possess sialidase activity. A potential reason why M. mulieris significantly decreased sialic acid could be due to it being able to catabolize sialic acid. Phenyllactate is a relatively understudied metabolite that was significantly upregulated by Eggerthella sp. infection (p=0.0028) and exhibited the largest fold change out of any metabolites detected in our data set (~1,150 fold). Overall, Eggerthella sp. infections significantly altered multiple metabolites related to BV symptoms, particularly biogenic amines and those linked to epithelial barrier function, such as sphingolipids and glycerolipids (Hannun and Obeid, 2008; Bittman, 2013; Jernigan et al., 2015). In contrast M. mulieris infections significantly increased metabolites related to energy metabolism and oxidative stress (Figure 7).
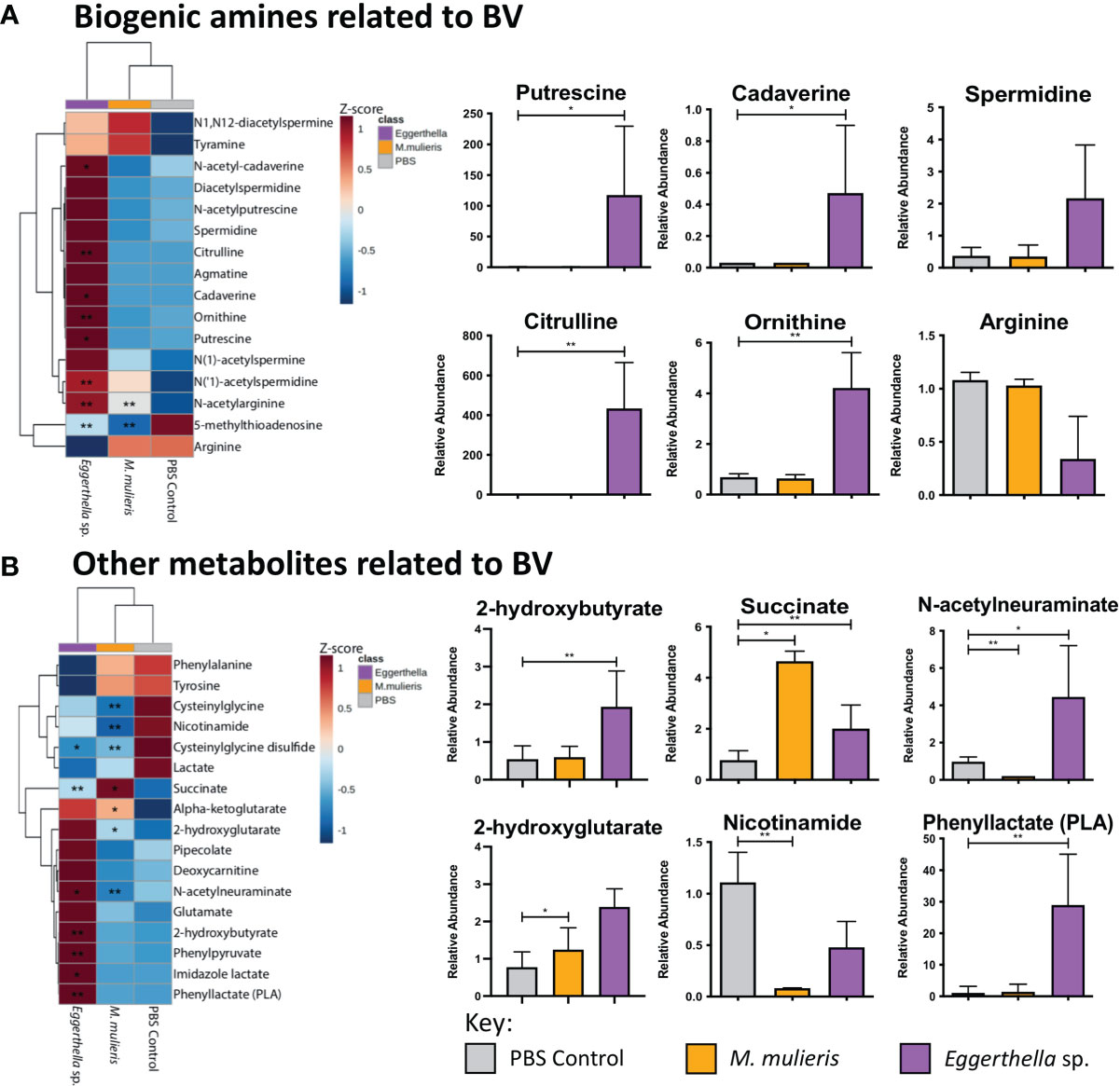
Figure 6 Eggerthella sp. significantly elevated the production of metabolites associated with biogenic amines and M. mulieris significantly altered metabolites related to energy metabolism. Hierarchical clustering analysis (HCA) and relative abundance of the BV-associated metabolites of Eggerthella sp. MVA1 and M. mulieris UPII-28I infection compared to the PBS mock-infected control: biogenic amines (A) and other metabolites related to BV symptoms (B). HCA was performed using Euclidean distance measures and average linkage clustering algorithms. Statistical significance was calculated using Student’s t-tests with Welch’s correction compared to PBS mock-infected control; *, p<0.05; **, p<0.01.
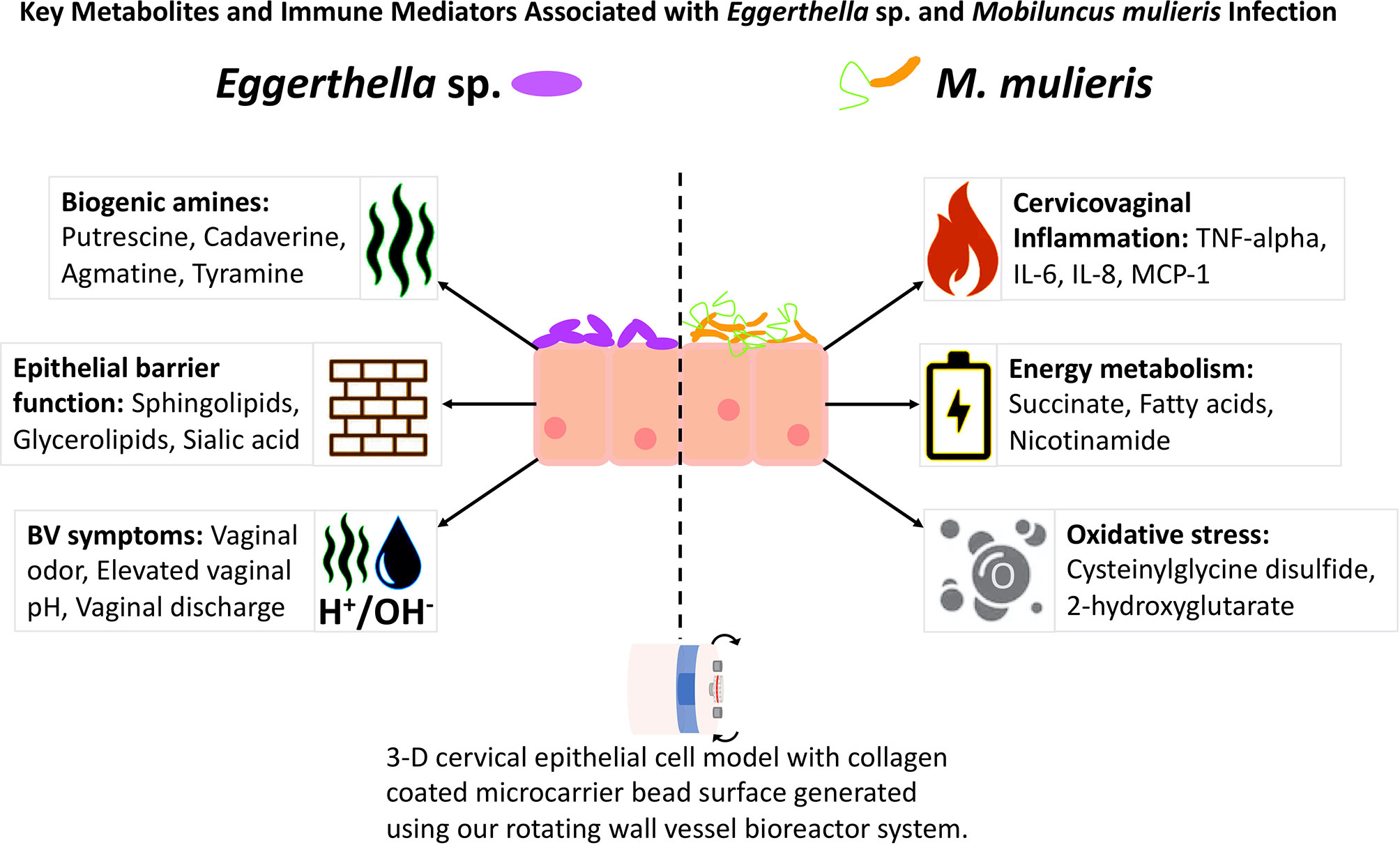
Figure 7 Comparisons of Eggerthella sp. and M. mulieris infections of 3-D cervical epithelial cell models. Supernatants from infections of 3-D cervical cell models with Eggerthella sp. MVA1 and M. mulieris UPII-28I were compared to PBS mock-infected controls in Bio-Plex and global metabolomics analyses. M. mulieris UPII-28I significantly increased inflammatory markers: IL-6 (p<0.0001), IL-8 (p<0.0001), MCP-1 (p<0.01) and TNF-α (p<0.01). Metabolites related to energy metabolism, such as nicotinamide (p=0.00429) and succinate (p=0.0335) were increased following M. mulieris UPII-28I infection but are relatively understudied in relation to BV. Furthermore, metabolites linked to oxidative stress, such as 2-hydroxyglutarate (p=0.0365), cysteinylglycine (p=0.00103) and cysteinylglycine disulfide (p=0.00238) were significantly altered by M. mulieris UPII-28I and are associated with inflammation. Eggerthella sp. MVA1 on the other hand significantly altered levels of the biogenic amines cadaverine (p=0.0373) and putrescine (p=0.0101) and elevated levels of sphingolipids, glycerolipids and sialic acid (p=0.0376), each of which are metabolites relating to the epithelial barrier function.
Discussion
BV is characterized by colonization of the cervicovaginal epithelium by a diverse community of anaerobic bacteria. M. mulieris and bacterial species from the family Eggerthellaceae are relatively understudied bacteria compared to many of the other BV-associated microorganisms. In this study we aimed to examine how two vaginal isolates: Eggerthella sp. strain MVA1 and M. mulieris strain UPII-28I, influence the immunometabolic landscape in the context of the lower FRT, as well as the potential pathophysiological contributions of these species to BV.
In recent years, there has been a reclassification of members belonging to the Eggerthellaceae family leading to questions related to the contributions of specific family members to the BV state. Although genomic information is limited for Eggerthella sp. strain MVA1, there is a sequence read SRX655730 available in the NCBI Sequence Read Archive. The 16S rRNA gene was sequenced from isolate Eggerthella sp. MVA1 (JX103988) and shares 99% sequence identity with Eggerthella lenta, however the species was not assigned by the BEI repository. There is very little information about E. lenta in the FRT and the vaginal microbiome since it is predominantly a gut microbe. E. lenta is found at low abundance in the FRT and may be transferred to the vagina from the gastrointestinal tract (Priputnevich et al., 2021). Unfortunately, until the comparative genomic analysis is performed, we cannot classify a species for the strain Eggerthella sp. MVA1. The family Eggerthellaceae also contains Coriobacteriales bacterium DNF00809, previously classified as Eggerthella sp. type 1 by Srinivasan et al. (Srinivasan et al., 2016) before culture and whole genome sequencing of the species. Previous studies showed that this bacterium was present in 85-95% of women with BV compared to those without BV (Fredricks et al., 2005; Fredricks et al., 2007; Srinivasan et al., 2012; Shipitsyna et al., 2013). These previous studies have also shown that E. lenta is much less prevalent in the FRT than Coriobacteriales bacterium DNF00809, which is no longer classified as an Eggerthella species. Although beyond the scope of this study, an in-depth analysis of taxonomic classification of vaginal bacteria belonging to the family Eggerthellaceae should be further investigated and clear taxonomic nomenclature should be referenced for this family to reflect the complexity of its lineage and putative role in BV and the cervicovaginal environment. Our study provides data related to the immunometabolic contributions with one of these understudied vaginal strains from the family Eggerthelleacae.
It is important to investigate BV-associated bacteria in the cervical epithelium since disruption of the microbiota at this mucosal site can lead to PTB, increased STI acquisition and other gynecological sequalae (Brunham and Paavonen, 2020). BV can also lead to ascension of pathogenic bacteria to the upper FRT, therefore promoting endometritis and pelvic inflammatory disease (Eckert et al., 2002). To determine the individual immunometabolic contributions of Eggerthella sp. and M. mulieris within the cervical microenvironment, we utilized a bioreactor-derived 3-D cervical epithelial cell model (Barrila et al., 2010; Hjelm et al., 2010; Gardner and Herbst-Kralovetz, 2016) in combination with multiplex immunoassays and global untargeted metabolomic approaches to identify key metabolites and immune mediators, respectively, related to M. mulieris and Eggerthella sp. infections.
Organotypic 3-D human cervical epithelial cell models recapitulate many features of parental tissue that are not observed in monolayer cell culture models. Our advanced 3-D cell culture model exhibits physiologically relevant features, such as TLR expression, microvilli, intercellular junctional complexes and secretory material that could influence how BV-associated bacteria can colonize the model in a way similar to in vivo tissues (Gardner et al., 2020; Łaniewski and Herbst-Kralovetz, 2021; Salliss et al., 2021) These features provide a more accurate representation of the in vivo state which is more amenable to translational research efforts and studying host-microbe interactions; however, each model system has its strengths, weaknesses and utility (Hjelm et al., 2010; Radtke and Herbst-Kralovetz, 2012; Radtke et al., 2012; Herbst-Kralovetz et al., 2016). The close relation of 3-D human cervical epithelial cells to cervical tissue allows us to investigate how these pathogens can cause pathophysiological changes to the cervicovaginal microenvironment and epithelia. Using SEM, we demonstrated colonization of the 3-D cervical epithelial cell model by Eggerthella sp. and M. mulieris (Figure 1). We evaluated the cytotoxicity of each bacterium and determined that neither Eggerthella sp. nor M. mulieris infections induced significant cytotoxicity in cervical cells.
Inflammation is a driver of many disease processes including BV where it has been previously associated with PTB (Meis et al., 1995; Goldenberg et al., 2000; Romero et al., 2007). Our immune mediator analysis revealed that both M. mulieris and Eggerthella sp. induced a proinflammatory response in 3-D cervical epithelial cell models. Eggerthella sp. significantly increased IL-1α while M. mulieris significantly elevated IL-6, IL-8, MCP-1 and TNFα. M. mulieris has been linked to the significant elevation of IL-6, IL-8 and TNFα in vitro (Anahtar et al., 2015; Dude et al., 2020). Flagella, such as those expressed by M. mulieris, have been linked to activation of TLR5 (Hayashi et al., 2001; Dela Cruz et al., 2021) which leads to the stimulation of the NF-κB signaling pathway and, in consequence, upregulation of IL-6, IL-8 and TNFα (Nasu and Narahara, 2010). Intriguingly, upregulation of IL-6 and IL-8 as well as the NF-κB signaling pathway have been connected to PTB (Romero et al., 2014). Clinical studies showed that IL-6, IL-8 and TNFα are some of the most common cytokines associated with PTB (Murtha et al., 1998; Coleman et al., 2001; Romero et al., 2014; Ville and Rozenberg, 2018). However, there is no clear diagnostic marker for PTB and proinflammatory cytokine and chemokine profiles differing among women who deliver pre-term (Wei et al., 2010; Fettweis et al., 2019). In addition, elevation of proinflammatory markers including IL-6 and IL-8 are associated with HIV infection risk (Mlisana et al., 2012; Rodriguez Garcia et al., 2015). Genital inflammation can lead to an increased risk of STI acquisition, with the infected epithelia being damaged, allowing the pathogens that cause STIs access to deeper tissues (Passmore et al., 2016). Inflammation of cervical and vaginal tissues induces recruitment of immune cells to the lower FRT which facilitates the spread of HIV and other STIs (Masson et al., 2014; Anahtar et al., 2015; Masson et al., 2015; Fichorova et al., 2020).
Oxidative stress has been closely linked to inflammation (Reuter et al., 2010). Oxidative stress becomes damaging when there are a disproportional amount of reactive oxygen species (ROS), that overwhelm the antioxidants capacity of glutathione (Schafer and Buettner, 2001). High levels of ROS can induce cellular damage and promote many inflammatory states including cancer (Perwez Hussain and Harris, 2007; Reuter et al., 2010; Burton and Jauniaux, 2011) by recruiting inflammatory markers such as cytokines and chemokines and stimulating NF-κB signaling (Reuter et al., 2010). In this study, we found that M. mulieris significantly altered multiple metabolites associated with oxidative stress, including cysteinylglycine, cysteinylglycine disulfide and 2-hydroxyglutarate, whilst Eggerthella sp. significantly altered 2-hydroxybutyrate and cysteinylglycine disulfide. Depletion of cysteinylglycine and cysteinylglycine disulfide, two intermediates in the glutathione synthesis pathway, could either indicate increased glutathione biosynthesis or signify an increase in the levels of ROS. Disruption of redox balance due to the elevated levels of ROS has been linked to activation of cell signaling pathways including those responsible for the regulation of inflammatory cytokines and PTB (Schieber and Chandel, 2014; Moore et al., 2018). Notably, increase of ROS can lead to lipid peroxidation which can free lipids from cell membranes (Burton and Jauniaux, 2011; Moore et al., 2018). The lipid peroxidation and membrane damage by ROS might be a mechanistic link behind the increased concentrations of lipids following infections of 3-D cervical models with Eggerthella sp. and M. mulieris.
Through our global untargeted metabolomics analyses, we found that Eggerthella sp. significantly altered twice as many lipids as M. mulieris. Specifically, sphingolipids were significantly elevated exclusively by Eggerthella sp. We also found similar significantly altered glycerolipids and sphingolipids as those reported by Salliss et al., 2021 that were elevated following infection with another BV-related microorganism: Megasphaera micronuciformis (Salliss et al., 2021). Sphingolipids are components of eukaryotic cell membranes and have been related to proinflammatory signaling pathways and apoptosis (Kolter and Sandhoff, 2006; Hannun and Obeid, 2008; Hannun and Obeid, 2018). Our results demonstrate that M. mulieris induced higher abundance of sphingolipids and most glycerolipids relative to Eggerthella sp. and PBS mock-infected controls, although the levels did not reach significance. We hypothesize that this may be related to the observation that M. mulieris has been shown to increase membrane permeability in cervical epithelial cells grown on transwells inserts (Dude et al., 2020) potentially by freeing these membrane-associated lipids. Both bacteria induced extracellular accumulation of multiple lipids related to epithelial barrier function (Bittman, 2013; Jernigan et al., 2015). Considering these results, we hypothesize that Eggerthella sp. and M. mulieris may play a role in increasing membrane permeability, although significant cytotoxicity was not observed in our experiments. Unexpectedly, compared to other lipids long-chain fatty acids (LCFAs) were significantly depleted by M. mulieris infection. It is possible that the depletion of LCFAs could result from an ability of M. mulieris to catabolize these liberated LCFAs a as an energy source. Unfortunately, the genomic sequence of M. mulieris is not fully annotated, therefore it is unclear if this bacterial species synthesizes all proteins necessary to facilitate the catabolism of the LCFAs.
The epithelial barrier function and the physiological properties of the mucosal membranes lining the FRT are crucial in protecting the cervix from BV-associated bacteria (Rodriguez Garcia et al., 2015). One of the key pathophysiological changes during BV is disruption of the epithelial barrier function, which allows pathogenic bacteria to access deeper tissues and induce inflammation (Muzny et al., 2019). Sialic acid is the terminal sugar moiety on glycans of cell surface glycoproteins and mucins. The epithelium of the FRT is lined with highly glycosylated mucins which limit adhesion and colonization of pathogenic bacteria during BV (Linden et al., 2008; Barrila et al., 2010; Lewis and Lewis, 2012; Radtke et al., 2012). In addition, sialic acid residues can bind to pathogens and induce host cell signaling to generate an immune response (Macauley et al., 2014; Bhide and Colley, 2017). Significant alterations in the levels of sialic acid following bacterial infections indicates bacteria-mediated sialidase activity. Previous clinical studies have revealed elevated levels of sialidase and sialic acid in the cervicovaginal fluids of women with BV (Briselden et al., 1992; Moncla et al., 2015). Infection of 3-D cervical cell models with both M. mulieris and Eggerthella sp. significantly altered the levels of sialic acid, indicating that both species exert sialidase activity. As M. mulieris decreased the levels of extracellular sialic acid, we hypothesize that this species catabolizes sialic acid residues that are liberated from the cell surfaces (Culhane et al., 2006), similarly to other BV-associated bacterium Gardnerella vaginalis (Lewis et al., 2013). The sialidase activity of M. mulieris could play a role in PTB since the mucus plug created during pregnancy contains multiple mucins which could be degraded by sialidase, therefore allowing pathogenic bacteria to ascend to the uterus (Mcgregor et al., 1994; Lewis and Lewis, 2012; Smith-Dupont et al., 2017; Baker et al., 2018). Consequently, ascension of pathogenic bacteria into the upper FRT during pregnancy can lead to chorioamnionitis and PTB (Galinsky et al., 2013). M. mulieris and elevated levels of IL-8 have been previously associated with amniotic infection and PID (Hillier et al., 1988; Larsson et al., 1989; Hitti et al., 2001).
Sialidase activity has been noted as a potential diagnostic marker for BV (Briselden et al., 1992) along with several cervicovaginal metabolites, some of which are highlighted by Srinivasan et al., (2015). Amongst the metabolites associated with BV, biogenic amines are also considered key players in many aspects of BV pathogenesis (Nelson et al., 2015; Srinivasan et al., 2015; Borgogna et al., 2021). The Amsel criteria and Nugent scores are two methods to diagnose BV (Amsel et al., 1983; Nugent et al., 1991). Putrescine and cadaverine have been linked to decreased in vitro growth of Lactobacillus spp. and high Nugent scores in women with BV (Borgogna et al., 2021). Both putrescine and cadaverine are associated with increased vaginal pH, vaginal amine odor and vaginal discharge that manifest during BV (Srinivasan et al., 2012; Yeoman et al., 2013; Nelson et al., 2015). Through our metabolomics analysis we found that Eggerthella sp. significantly elevated both putrescine and cadaverine in the extracellular milieu, which corresponds with observations from Srinivasan et al. (Srinivasan et al., 2012). In contrast, M. mulieris did not elevate any biogenic amines in our current study. Previously, M. mulieris has been linked to elevated trimethylamine (Spiegel, 1991; Africa et al., 2014), however, this polyamine was not detected in our metabolomics analysis. Phenyllactate was found to be significantly elevated for Eggerthella sp. with the highest fold change (1150-fold) of all the metabolites measured. Although the role of this metabolite in the cervicovaginal microenvironment is still not clear, we have previously observed its accumulation following infections with other vaginal bacteria (Łaniewski and Herbst-Kralovetz, 2021; Salliss et al., 2021). The severity of BV has been associated with increased risk of HIV and other STI acquisition (Allsworth and Peipert, 2011); thus, contribution of Eggerthella spp. and M. mulieris to clinical symptoms of BV mechanistically links these species to poor health outcomes related to BV.
As with all experiments and biological models there are limitations (Herbst-Kralovetz et al., 2016). The 3-D cell culture model we have used is a robust tool that can provide mechanistic insights into host-microbe interactions in the cervical microenvironment. Our model, as with most human in vitro cell culture models, requires the use of pH-buffered medium; thus, it cannot mimic the acidic pH found in healthy women in vivo without impacting cellular viability However, the BVAB tested in this study thrive in a more neutral pH environment, which is a characteristic of our model (Barrila et al., 2010; Hjelm et al., 2010; Gardner and Herbst-Kralovetz, 2016). We also acknowledge that the bacterial strains used in this study may not represent the other closely related strains or species. As stated previously, the taxonomy of Eggerthella sp. MVA1 is still incomplete; therefore, we cannot generalize our findings to the other members of the Eggerthellaceae family. Mobiluncus mulieris is closely related to Mobiluncus curtsii; however, the two species are unique from each other in terms of physical characteristics and enzymatic activity. M. curtsii is smaller in size, can hydrolyze starch and hippurate and produce citrulline, ornithine and ammonia from arginine whilst M. mulieris cannot (Spiegel and Roberts, 1984). Future studies utilizing additional well-characterized bacterial isolates in mono- or polymicrobial infections are needed to better understand the individual contributions of these BVAB to poor gynecologic and obstetric outcomes.
Overall, we found Eggerthella sp. infections significantly altered multiple metabolites related to BV symptoms. These metabolites included the biogenic amines putrescine and cadaverine, as well as their precursors, and metabolites linked to epithelial barrier function, such as sphingolipids and glycerolipids (Ghosh et al., 1997; Bittman, 2013; Jernigan et al., 2015; Hannun and Obeid, 2018; Harrison et al., 2018; Heaver et al., 2018). M. mulieris infections significantly elevated multiple proinflammatory markers that are linked to PTB in addition to metabolites related to energy metabolism and oxidative stress (Figure 7). This study sheds light into the mechanisms that Eggerthella sp. and M. mulieris may utilize to promote BV. Our data suggests that Eggerthella sp. plays a key role in the production of biogenic amines, which contribute to the elevated vaginal pH and the amine odor, whilst M. mulieris potentially impacts the membrane permeability and induce proinflammatory immune responses. The increased concentration of lipids present in M. mulieris infection could also link into the increased immune response (Hannun and Obeid, 2018; Albeituni and Stiban, 2019; Sukocheva et al., 2020). The link to inflammation and the altered metabolic microenvironment fits into the hypothesis of Muzny et al. (2020) that proposes early colonizers establish biofilm and evade host defense responses whereas secondary colonizers mediate inflammation, an altered metabolic microenvironment and symptoms associated with BV. Based on our data, we propose that M. mulieris is functioning as a secondary colonizer in this hypothetical model of BV. In contrast, Eggerthella sp. while not inflammatory, exhibits metabolic activity consistent with our definition of a secondary colonizer, but may also participate in the early stages of biofilm formation. However, further in vitro studies investigating these microorganisms in polymicrobial settings in conjunction with longitudinal clinical studies are needed to elucidate microbe-microbe interactions and determine the role of these bacteria in the context of BV biofilms.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
MH-K, PŁ, and JM conceived of the experimental design and interpretation of the data. JM conducted experimental infections of the 3-D cervical cell aggregates and the Bio-Plex analyses. Cell supernatants were sent to Metabolon, Inc. for global untargeted metabolomics analysis. RM carried out the cytotoxicity experiments as well as the analysis of the cytotoxicity data, Bio-Plex and metabolomics data. RM was also responsible for writing the first draft of the manuscript, drafting and editing the figures and revising the manuscript. JM obtained SEM images of infected 3-D human cervical cell models and assisted in the statistical analysis. MH-K, JM, and PŁ provided support and advice on writing, figures and tables, and also read and revised the manuscript. MH-K and PŁ provided guidance of the experimental and writing processes. MH-K supervised the research and provided funding acquisition, project administration and resources. All authors read, revised, and approved the final version of the manuscript.
Funding
The funding for this study was supplied by the NIH National Cancer Institute (3P30CA023074-39S3) and the Flinn Foundation (2244) to MH-K.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Dr. Nicole Jimenez for critical review of the manuscript. We would also like to thank David Lowry at Arizona State University for his contributions in SEM sample preparation and imaging. Furthermore, we would like to recognize the Biodefense and Emerging Infections Research Resources Repository (https://www.beiresources.org/Home.aspx) for supplying the bacterial isolates for this research; M. mulieris UPII-28I and Eggerthella sp. MVA1.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.759697/full#supplementary-material
References
Africa, C. W. J., Nel, J., Stemmet, M. (2014). Anaerobes and Bacterial Vaginosis in Pregnancy: Virulence Factors Contributing to Vaginal Colonisation. Int. J. Environ. Res. Public Health 11, 6979–7000. doi: 10.3390/ijerph110706979
Albeituni, S., Stiban, J. (2019). Roles of Ceramides and Other Sphingolipids in Immune Cell Function and Inflammation. Adv. Exp. Med. Biol. 1161, 169–191. doi: 10.1007/978-3-030-21735-8_15
Allsworth, J. E., Peipert, J. F. (2011). Severity of Bacterial Vaginosis and the Risk of Sexually Transmitted Infection. Am. J. Obstet. Gynecol. 205, 113.e111–113.e1136. doi: 10.1016/j.ajog.2011.02.060
Amsel, R., Totten, P. A., Spiegel, C. A., Chen, K. C., Eschenbach, D., Holmes, K. K. (1983). Nonspecific Vaginitis. Diagnostic Criteria and Microbial and Epidemiologic Associations. Am. J. Med. 74, 14–22. doi: 10.1016/0002-9343(83)91112-9
Anahtar, M., Byrne, E., Doherty, K., Bowman, B., Yamamoto, H., Soumillon, M., et al. (2015). Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity 42, 965–976. doi: 10.1016/j.immuni.2015.04.019
Baker, J. M., Chase, D. M., Herbst-Kralovetz, M. M. (2018). Uterine Microbiota: Residents, Tourists, or Invaders? Front. Immunol. 9, 208. doi: 10.3389/fimmu.2018.00208
Barrila, J., Radtke, A. L., Crabbé, A., Sarker, S. F., Herbst-Kralovetz, M. M., Ott, C. M., et al. (2010). Organotypic 3D Cell Culture Models: Using the Rotating Wall Vessel to Study Host–Pathogen Interactions. Nat. Rev. Microbiol. 8, 791–801. doi: 10.1038/nrmicro2423
Bhide, G. P., Colley, K. J. (2017). Sialylation of N-Glycans: Mechanism, Cellular Compartmentalization and Function. Histochem. Cell Biol. 147, 149–174. doi: 10.1007/s00418-016-1520-x
Bittman, R. (2013). “Glycerolipids: Chemistry,” in Encyclopedia of Biophysics. Ed. Roberts, G. C. K. (Berlin, Heidelberg: Springer Berlin Heidelberg), 907–914.
Borgogna, J. C., Shardell, M. D., Grace, S. G., Santori, E. K., Americus, B., Li, Z., et al. (2021). Biogenic Amines Increase the Odds of Bacterial Vaginosis and Affect the Growth of and Lactic Acid Production by Vaginal Lactobacillus Spp. Appl. Environ. Microbiol. 87, e03068–20. doi: 10.1128/AEM.03068-20
Briselden, A. M., Moncla, B. J., Stevens, C. E., Hillier, S. L. (1992). Sialidases (Neuraminidases) in Bacterial Vaginosis and Bacterial Vaginosis-Associated Microflora. J. Clin. Microbiol. 30, 663–666. doi: 10.1128/jcm.30.3.663-666.1992
Brunham, R. C., Paavonen, J. (2020). Reproductive System Infections in Women: Upper Genital Tract, Fetal, Neonatal and Infant Syndromes. Pathog. Dis. 78, ftaa023. doi: 10.1093/femspd/ftaa023
Buckner, L. R., Schust, D. J., Ding, J., Nagamatsu, T., Beatty, W., Chang, T. L., et al. (2011). Innate Immune Mediator Profiles and Their Regulation in a Novel Polarized Immortalized Epithelial Cell Model Derived From Human Endocervix. J. Reprod. Immunol. 92, 8–20. doi: 10.1016/j.jri.2011.08.002
Burton, G. J., Jauniaux, E. (2011). Oxidative Stress. Best Pract. Res. Clin. Obstet. Gynaecol. 25, 287–299. doi: 10.1016/j.bpobgyn.2010.10.016
Coleman, M., Keelan, J. A., Mccowan, L. M. E., Townend, K. M., Mitchell, M. D. (2001). Predicting Preterm Delivery: Comparison of Cervicovaginal Interleukin (IL)-1β, IL-6 and IL-8 With Fetal Fibronectin and Cervical Dilatation. Eur. J. Obstet. Gynecol. Reprod. Biol. 95, 154–158. doi: 10.1016/S0301-2115(00)00450-4
Critchfield, A. S., Yao, G., Jaishankar, A., Friedlander, R. S., Lieleg, O., Doyle, P. S., et al. (2013). Cervical Mucus Properties Stratify Risk for Preterm Birth. PloS One 8, e69528. doi: 10.1371/journal.pone.0069528
Culhane, J. F., Nyirjesy, P., Mccollum, K., Goldenberg, R. L., Gelber, S. E. (2006). Variation in Vaginal Immune Parameters and Microbial Hydrolytic Enzymes in Bacterial Vaginosis Positive Pregnant Women With and Without Mobiluncus Species. Am. J. Obstet. Gynecol. 195, 516–521. doi: 10.1016/j.ajog.2006.02.036
Danielsson, D., Teigen, P. K., Moi, H. (2011). The Genital Econiche: Focus on Microbiota and Bacterial Vaginosis. Ann. N. Y. Acad. Sci. 1230, 48–58. doi: 10.1111/j.1749-6632.2011.06041.x
Dela Cruz, E. J., Fiedler, T. L., Liu, C., Munch, M. M., Kohler, C. M., Oot, A. R., et al. (2021). Genetic Variation in Toll-Like Receptor 5 and Colonization With Flagellated Bacterial Vaginosis-Associated Bacteria. Infect. Immun. 89, e00060–e00020. doi: 10.1128/IAI.00060-20
Dude, C. M., Saylany, A., Brown, A., Elovitz, M., Anton, L. (2020). Microbial Supernatants From Mobiluncus Mulieris, a Bacteria Strongly Associated With Spontaneous Preterm Birth, Disrupts the Cervical Epithelial Barrier Through Inflammatory and miRNA Mediated Mechanisms. Anaerobe 61, 102127. doi: 10.1016/j.anaerobe.2019.102127
Eckert, L. O., Hawes, S. E., Wölner-Hanssen, P. K., Kiviat, N. B., Wasserheit, J. N., Paavonen, J. A., et al. (2002). Endometritis: The Clinical-Pathologic Syndrome. Am. J. Obstet. Gynecol. 186, 690–695. doi: 10.1067/mob.2002.121728
Fettweis, J. M., Serrano, M. G., Brooks, J. P., Edwards, D. J., Girerd, P. H., Parikh, H. I., et al. (2019). The Vaginal Microbiome and Preterm Birth. Nat. Med. 25, 1012–1021. doi: 10.1038/s41591-019-0450-2
Fichorova, R. N., Morrison, C. S., Chen, P.-L., Yamamoto, H. S., Govender, Y., Junaid, D., et al. (2020). Aberrant Cervical Innate Immunity Predicts Onset of Dysbiosis and Sexually Transmitted Infections in Women of Reproductive Age. PloS One 15, e0224359. doi: 10.1371/journal.pone.0224359
Finegold, S. M., Sutter, V. L., Mathisen, G. E. (1983). “Normal Indigenous Intestinal Flora,” in Human Intestinal Microflora in Health and Disease Cambridge, Massachusetts: Academic Press 1. pp. 3–31.
Fredricks, D. N., Fiedler, T. L., Marrazzo, J. M. (2005). Molecular Identification of Bacteria Associated With Bacterial Vaginosis. N. Engl. J. Med. 353, 1899–1911. doi: 10.1056/NEJMoa043802
Fredricks, D. N., Fiedler, T. L., Thomas, K. K., Oakley, B. B., Marrazzo, J. M. (2007). Targeted PCR for Detection of Vaginal Bacteria Associated With Bacterial Vaginosis. J. Clin. Microbiol. 45, 3270–3276. doi: 10.1128/JCM.01272-07
Galinsky, R., Polglase, G. R., Hooper, S. B., Black, M. J., Moss, T. J. M. (2013). The Consequences of Chorioamnionitis: Preterm Birth and Effects on Development. J. Pregnancy 2013, 412831. doi: 10.1155/2013/412831
Gardner, J. K., Herbst-Kralovetz, M. M. (2016). Three-Dimensional Rotating Wall Vessel-Derived Cell Culture Models for Studying Virus-Host Interactions. Viruses 8, 304. doi: 10.3390/v8110304
Gardner, J. K., Łaniewski, P., Knight, A., Haddad, L. B., Swaims-Kohlmeier, A., Herbst-Kralovetz, M. M. (2020). Interleukin-36γ Is Elevated in Cervicovaginal Epithelial Cells in Women With Bacterial Vaginosis and In Vitro After Infection With Microbes Associated With Bacterial Vaginosis. J. Infect. Dis. 221, 983–988. doi: 10.1093/infdis/jiz514
Gatti, M. (2000). Isolation of Mobiluncus Species From the Human Vagina. Zentralbl. Bakteriol. 289, 869–878. doi: 10.1016/S0934-8840(00)80017-1
Ghosh, S., Strum, J. C., Bell, R. M. (1997). Lipid Biochemistry: Functions of Glycerolipids and Sphingolipids in Cellular Signaling. FASEB J. 11, 45–50. doi: 10.1096/fasebj.11.1.9034165
Glupczynski, Y., Labbé, M., Crokaert, F., Pepersack, F., van der Auwera, P., Yourassowsky, E. (1984). Isolation of Mobiluncus in Four Cases of Extragenital Infections in Adult Women. Eur. J. Clin. Microbiol. 3, 433–435. doi: 10.1007/BF02017365
Goldenberg, R. L., Hauth, J. C., Andrews, W. W. (2000). Intrauterine Infection and Preterm Delivery. N. Engl. J. Med. 342, 1500–1507. doi: 10.1056/NEJM200005183422007
Hallén, A., Påhlson, C., Forsum, U. (1987). Bacterial Vaginosis in Women Attending STD Clinic: Diagnostic Criteria and Prevalence of Mobiluncus Spp. Genitourin. Med. 63, 386–389. doi: 10.1136/sti.63.6.386
Hannun, Y. A., Obeid, L. M. (2008). Principles of Bioactive Lipid Signalling: Lessons From Sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150. doi: 10.1038/nrm2329
Hannun, Y. A., Obeid, L. M. (2018). Sphingolipids and Their Metabolism in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 19, 175–191. doi: 10.1038/nrm.2017.107
Harrison, P. J., Dunn, T. M., Campopiano, D. J. (2018). Sphingolipid Biosynthesis in Man and Microbes. Nat. Prod. Rep. 35, 921–954. doi: 10.1039/C8NP00019K
Hayashi, F., Smith, K. D., Ozinsky, A., Hawn, T. R., Yi, E. C., Goodlett, D. R., et al. (2001). The Innate Immune Response to Bacterial Flagellin Is Mediated by Toll-Like Receptor 5. Nature 410, 1099–1103. doi: 10.1038/35074106
Heaver, S. L., Johnson, E. L., Ley, R. E. (2018). Sphingolipids in Host-Microbial Interactions. Curr. Opin. Microbiol. 43, 92–99. doi: 10.1016/j.mib.2017.12.011
Herbst-Kralovetz, M. M., Pyles, R. B., Ratner, A. J., Sycuro, L. K., Mitchell, C. (2016). New Systems for Studying Intercellular Interactions in Bacterial Vaginosis. J. Infect. Dis. 214 Suppl 1, S6–S13. doi: 10.1093/infdis/jiw130
Herbst-Kralovetz, M. M., Quayle, A. J., Ficarra, M., Greene, S., Rose, W. A., 32nd, Chesson, R., et al. (2008). Quantification and Comparison of Toll-Like Receptor Expression and Responsiveness in Primary and Immortalized Human Female Lower Genital Tract Epithelia. Am. J. Reprod. Immunol. 59, 212–224. doi: 10.1111/j.1600-0897.2007.00566.x
Hillier, S. L., Martius, J., Krohn, M., Kiviat, N., Holmes, K. K., Eschenbach, D. A. (1988). A Case–Control Study of Chorioamnionic Infection and Histologic Chorioamnionitis in Prematurity. N. Engl. J. Med. 319, 972–978. doi: 10.1056/NEJM198810133191503
Hillier, S. L., Nugent, R. P., Eschenbach, D. A., Krohn, M. A., Gibbs, R. S., Martin, D. H., et al. (1995). Association Between Bacterial Vaginosis and Preterm Delivery of a Low-Birth-Weight Infant. The Vaginal Infections and Prematurity Study Group. N. Engl. J. Med. 333, 1737–1742. doi: 10.1056/NEJM199512283332604
Hitti, J., Hillier, S. L., Agnew, K. J., Krohn, M. A., Reisner, D. P., Eschenbach, D. A. (2001). Vaginal Indicators of Amniotic Fluid Infection in Preterm Labor. Obstet. Gynecol. 97, 211–219. doi: 10.1016/s0029-7844(00)01146-7
Hjelm, B. E., Berta, A. N., Nickerson, C. A., Arntzen, C. J., Herbst-Kralovetz, M. M. (2010). Development and Characterization of a Three-Dimensional Organotypic Human Vaginal Epithelial Cell Model. Biol. Reprod. 82, 617–627. doi: 10.1095/biolreprod.109.080408
Holst, E., Goffeng, A. R., Andersch, B. (1994). Bacterial Vaginosis and Vaginal Microorganisms in Idiopathic Premature Labor and Association With Pregnancy Outcome. J. Clin. Microbiol. 32, 176–186. doi: 10.1128/jcm.32.1.176-186.1994
Ilhan, Z. E., Łaniewski, P., Tonachio, A., Herbst-Kralovetz, M. M. (2020). Members of Prevotella Genus Distinctively Modulate Innate Immune and Barrier Functions in a Human Three-Dimensional Endometrial Epithelial Cell Model. J. Infect. Dis. 222, 2082–2092. doi: 10.1093/infdis/jiaa324
Jackson, R., Maarsingh, J. D., Herbst-Kralovetz, M. M., Van Doorslaer, K. (2020). 3D Oral and Cervical Tissue Models for Studying Papillomavirus Host-Pathogen Interactions. Curr. Protoc. Microbiol. 59, e129. doi: 10.1002/cpmc.129
Jernigan, P. L., Makley, A. T., Hoehn, R. S., Edwards, M. J., Pritts, T. A. (2015). The Role of Sphingolipids in Endothelial Barrier Function. Biol. Chem. 396, 681–691. doi: 10.1515/hsz-2014-0305
Kageyama, A., Benno, Y., Nakase, T. (1999). Phylogenetic Evidence for the Transfer of Eubacterium Lentum to the Genus Eggerthella as Eggerthella Lenta Gen. Nov., Comb. Nov. Int. J. Syst. Bacteriol. 49 Pt 4, 1725–1732. doi: 10.1099/00207713-49-4-1725
Katz, K. S., Shutov, O., Lapoint, R., Kimelman, M., Brister, J. R., O'sullivan, C. (2021). STAT: A Fast, Scalable, MinHash-Based K-Mer Tool to Assess Sequence Read Archive Next-Generation Sequence Submissions. Genome Biol. 22, 270. doi: 10.1186/s13059-021-02490-0
Kolter, T., Sandhoff, K. (2006). Sphingolipid Metabolism Diseases. Biochim. Biophys. Acta (BBA) - Biomembranes 1758, 2057–2079. doi: 10.1016/j.bbamem.2006.05.027
Łaniewski, P., Barnes, D., Goulder, A., Cui, H., Roe, D. J., Chase, D. M., et al. (2018). Linking Cervicovaginal Immune Signatures, HPV and Microbiota Composition in Cervical Carcinogenesis in non-Hispanic and Hispanic Women. Sci. Rep. 8, 7593. doi: 10.1038/s41598-018-25879-7
Łaniewski, P., Herbst-Kralovetz, M. M. (2021). Bacterial Vaginosis and Health-Associated Bacteria Modulate the Immunometabolic Landscape in 3D Model of Human Cervix. NPJ Biofilms Microbiomes. accepted for publication. doi: 10.1038/s41522-021-00259-8
Larsson, P. G., Bergman, B., Forsum, U., Platz-Christensen, J. J., Påhlson, C. (1989). Mobiluncus and Clue Cells as Predictors of PID After First-Trimester Abortion. Acta Obstet. Gynecol. Scand. 68, 217–220. doi: 10.3109/00016348909020992
Lau, S. K., Woo, P. C., Fung, A. M., Chan, K. M., Woo, G. K., Yuen, K. Y. (2004a). Anaerobic, non-Sporulating, Gram-Positive Bacilli Bacteraemia Characterized by 16S rRNA Gene Sequencing. J. Med. Microbiol. 53, 1247–1253. doi: 10.1099/jmm.0.45803-0
Lau, S. K., Woo, P. C., Woo, G. K., Fung, A. M., Wong, M. K., Chan, K. M., et al. (2004b). Eggerthella Hongkongensis Sp. Nov. And Eggerthella Sinensis Sp. Nov., Two Novel Eggerthella Species, Account for Half of the Cases of Eggerthella Bacteremia. Diagn. Microbiol. Infect. Dis. 49, 255–263. doi: 10.1016/j.diagmicrobio.2004.04.012
Lee, M. R., Huang, Y. T., Liao, C. H., Chuang, T. Y., Wang, W. J., Lee, S. W., et al. (2012). Clinical and Microbiological Characteristics of Bacteremia Caused by Eggerthella, Paraeggerthella, and Eubacterium Species at a University Hospital in Taiwan From 2001 to 2010. J. Clin. Microbiol. 50, 2053–2055. doi: 10.1128/JCM.00548-12
Lewis, A. L., Lewis, W. G. (2012). Host Sialoglycans and Bacterial Sialidases: A Mucosal Perspective. Cell. Microbiol. 14, 1174–1182. doi: 10.1111/j.1462-5822.2012.01807.x
Lewis, W. G., Robinson, L. S., Gilbert, N. M., Perry, J. C., Lewis, A. L. (2013). Degradation, Foraging, and Depletion of Mucus Sialoglycans by the Vagina-Adapted Actinobacterium Gardnerella Vaginalis. J. Biol. Chem. 288, 12067–12079. doi: 10.1074/jbc.M113.453654
Linden, S. K., Sutton, P., Karlsson, N. G., Korolik, V., Mcguckin, M. A. (2008). Mucins in the Mucosal Barrier to Infection. Mucosal Immunol. 1, 183–197. doi: 10.1038/mi.2008.5
Macauley, M. S., Crocker, P. R., Paulson, J. C. (2014). Siglec-Mediated Regulation of Immune Cell Function in Disease. Nat. Rev. Immunol. 14, 653–666. doi: 10.1038/nri3737
Machado, A., Cerca, N. (2015). Influence of Biofilm Formation by Gardnerella Vaginalis and Other Anaerobes on Bacterial Vaginosis. J. Infect. Dis. 212, 1856–1861. doi: 10.1093/infdis/jiv338
Masson, L., Mlisana, K., Little, F., Werner, L., Mkhize, N. N., Ronacher, K., et al. (2014). Defining Genital Tract Cytokine Signatures of Sexually Transmitted Infections and Bacterial Vaginosis in Women at High Risk of HIV Infection: A Cross-Sectional Study. Sex. Transm. Infect. 90, 580. doi: 10.1136/sextrans-2014-051601
Masson, L., Passmore, J. A., Liebenberg, L. J., Werner, L., Baxter, C., Arnold, K. B., et al. (2015). Genital Inflammation and the Risk of HIV Acquisition in Women. Clin. Infect. Dis. 61, 260–269. doi: 10.1093/cid/civ298
Mcgowin, C. L., Radtke, A. L., Abraham, K., Martin, D. H., Herbst-Kralovetz, M. (2013). Mycoplasma Genitalium Infection Activates Cellular Host Defense and Inflammation Pathways in a 3-Dimensional Human Endocervical Epithelial Cell Model. J. Infect. Dis. 207, 1857–1868. doi: 10.1093/infdis/jit101
Mcgregor, J. A., French, J. I., Jones, W., Milligan, K., Mckinney, P. J., Patterson, E., et al. (1994). Bacterial Vaginosis Is Associated With Prematurity and Vaginal Fluid Mucinase and Sialidase: Results of a Controlled Trial of Topical Clindamycin Cream. Am. J. Obstet. Gynecol. 170, 1048–1059; discussion 1059-1060. doi: 10.1016/S0002-9378(94)70098-2
Meis, P. J., Goldenberg, R. L., Mercer, B., Moawad, A., Das, A., Mcnellis, D., et al. (1995). The Preterm Prediction Study: Significance of Vaginal Infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am. J. Obstet. Gynecol. 173, 1231–1235. doi: 10.1016/0002-9378(95)91360-2
Metsalu, T., Vilo, J. (2015). ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data Using Principal Component Analysis and Heatmap. Nucleic Acids Res. 43, W566–W570. doi: 10.1093/nar/gkv468
Mlisana, K., Naicker, N., Werner, L., Roberts, L., Van Loggerenberg, F., Baxter, C., et al. (2012). Symptomatic Vaginal Discharge Is a Poor Predictor of Sexually Transmitted Infections and Genital Tract Inflammation in High-Risk Women in South Africa. J. Infect. Dis. 206, 6–14. doi: 10.1093/infdis/jis298
Moi, H., Fredlund, H., Törnqvist, E., Danielsson, D. (1991). Mobiluncus Species in Bacterial Vaginosis: Aspects of Pathogenesis. Apmis 99, 1049–1054. doi: 10.1111/j.1699-0463.1991.tb01298.x
Moncla, B. J., Chappell, C. A., Mahal, L. K., Debo, B. M., Meyn, L. A., Hillier, S. L. (2015). Impact of Bacterial Vaginosis, as Assessed by Nugent Criteria and Hormonal Status on Glycosidases and Lectin Binding in Cervicovaginal Lavage Samples. PloS One 10, e0127091–e0127091. doi: 10.1371/journal.pone.0127091
Moore, T. A., Ahmad, I. M., Zimmerman, M. C. (2018). Oxidative Stress and Preterm Birth: An Integrative Review. Biol. Res. Nurs. 20, 497–512. doi: 10.1177/1099800418791028
Murtha, A. P., Greig, P. C., Jimmerson, C. E., Herbert, W. N. P. (1998). Maternal Serum Interleukin-6 Concentration as a Marker for Impending Preterm Delivery. Obstet. Gynecol. 91, 161–164. doi: 10.1016/S0029-7844(97)00602-9
Muzny, C. A., Łaniewski, P., Schwebke, J. R., Herbst-Kralovetz, M. M. (2020). Host-Vaginal Microbiota Interactions in the Pathogenesis of Bacterial Vaginosis. Curr. Opin. Infect. Dis. 33, 59–65. doi: 10.1097/QCO.0000000000000620
Muzny, C. A., Taylor, C. M., Swords, W. E., Tamhane, A., Chattopadhyay, D., Cerca, N., et al. (2019). An Updated Conceptual Model on the Pathogenesis of Bacterial Vaginosis. J. Infect. Dis. 220, 1399–1405. doi: 10.1093/infdis/jiz342
Nasu, K., Narahara, H. (2010). Pattern Recognition via the Toll-Like Receptor System in the Human Female Genital Tract. Mediators Inflamm. 2010, 976024–976024. doi: 10.1155/2010/976024
Nelson, T. M., Borgogna, J.-L. C., Brotman, R. M., Ravel, J., Walk, S. T., Yeoman, C. J. (2015). Vaginal Biogenic Amines: Biomarkers of Bacterial Vaginosis or Precursors to Vaginal Dysbiosis? Front. Physiol. 6, 253. doi: 10.3389/fphys.2015.00253
Nugent, R. P., Krohn, M. A., Hillier, S. L. (1991). Reliability of Diagnosing Bacterial Vaginosis Is Improved by a Standardized Method of Gram Stain Interpretation. J. Clin. Microbiol. 29, 297–301. doi: 10.1128/jcm.29.2.297-301.1991
O’hanlon, D. E., Moench, T. R., Cone, R. A. (2011). In Vaginal Fluid, Bacteria Associated With Bacterial Vaginosis Can Be Suppressed With Lactic Acid But Not Hydrogen Peroxide. BMC Infect. Dis. 11, 200. doi: 10.1186/1471-2334-11-200
Onderdonk, A. B., Delaney, M. L., Fichorova, R. N. (2016). The Human Microbiome During Bacterial Vaginosis. Clin. Microbiol. Rev. 29, 223–238. doi: 10.1128/CMR.00075-15
Pang, Z., Chong, J., Zhou, G., De lima morais, D. A., Chang, L., Barrette, M., et al. (2021). MetaboAnalyst 5.0: Narrowing the Gap Between Raw Spectra and Functional Insights. Nucleic Acids Res. 49, W388–W396. doi: 10.1093/nar/gkab382
Passmore, J.-a., Jaspan, H. B., Masson, L. (2016). Genital Inflammation, Immune Activation and Risk of Sexual HIV Acquisition. Curr. Opin. HIV AIDS 11, 156–162. doi: 10.1097/COH.0000000000000232
Perwez Hussain, S., Harris, C. C. (2007). Inflammation and Cancer: An Ancient Link With Novel Potentials. Int. J. Cancer 121, 2373–2380. doi: 10.1002/ijc.23173
Priputnevich, T., Lyubasovskaya, L., Muravieva, V., Kondrakhin, A., Ignateva, A., Gordeev, A., et al. (2021). Postpartum Endometritis and Obstetrical Sepsis Associated With Eggerthella Lenta. Case Report and Review of the Literature. J. Matern. Fetal Neonatal Med. 34, 313–317. doi: 10.1080/14767058.2019.1602602
Racicot, K., Cardenas, I., Wünsche, V., Aldo, P., Guller, S., Means, R. E., et al. (2013). Viral Infection of the Pregnant Cervix Predisposes to Ascending Bacterial Infection. J. Immunol. 191, 934. doi: 10.4049/jimmunol.1300661
Radtke, A. L., Herbst-Kralovetz, M. M. (2012). Culturing and Applications of Rotating Wall Vessel Bioreactor Derived 3D Epithelial Cell Models. J. Vis. Exp (62), e3868. doi: 10.3791/3868
Radtke, A. L., Quayle, A. J., Herbst-Kralovetz, M. M. (2012). Microbial Products Alter the Expression of Membrane-Associated Mucin and Antimicrobial Peptides in a Three-Dimensional Human Endocervical Epithelial Cell Model. Biol. Reprod. 87, 132. doi: 10.1095/biolreprod.112.103366
Reuter, S., Gupta, S. C., Chaturvedi, M. M., Aggarwal, B. B. (2010). Oxidative Stress, Inflammation, and Cancer: How are They Linked? Free Radical Biol. Med. 49, 1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006
Roberts, M. C., Hillier, S. L., Schoenknecht, F. D., Holmes, K. K. (1985). Comparison of Gram Stain, DNA Probe, and Culture for the Identification of Species of Mobiluncus in Female Genital Specimens. J. Infect. Dis. 152, 74–77. doi: 10.1093/infdis/152.1.74
Rodriguez Garcia, M., Patel, M. V., Shen, Z., Fahey, J. V., Biswas, N., Mestecky, J., et al. (2015). “Chapter 108 - Mucosal Immunity in the Human Female Reproductive Tract,” in Mucosal Immunology, 4th ed. Eds. Mestecky, J., Strober, W., Russell, M. W., Kelsall, B. L., Cheroutre, H., Lambrecht, B. N. (Boston: Academic Press), 2097–2124.
Romero, R., Dey, S. K., Fisher, S. J. (2014). Preterm Labor: One Syndrome, Many Causes. Science 345, 760–765. doi: 10.1126/science.1251816
Romero, R., Gotsch, F., Pineles, B., Kusanovic, J. P. (2007). Inflammation in Pregnancy: Its Roles in Reproductive Physiology, Obstetrical Complications, and Fetal Injury. Nutr. Rev. 65, S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x
Salliss, M. E., Maarsingh, J. D., Garza, C., Łaniewski, P., Herbst-Kralovetz, M. M. (2021). Veillonellaceae Family Members Uniquely Alter the Cervical Metabolic Microenvironment in a Human Three-Dimensional Epithelial Model. NPJ Biofilms Microbiomes 7, 57. doi: 10.1038/s41522-021-00229-0
Schafer, F. Q., Buettner, G. R. (2001). Redox Environment of the Cell as Viewed Through the Redox State of the Glutathione Disulfide/Glutathione Couple. Free Radic. Biol. Med. 30, 1191–1212. doi: 10.1016/S0891-5849(01)00480-4
Schieber, M., Chandel, N. S. (2014). ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 24, R453–R462. doi: 10.1016/j.cub.2014.03.034
Schwiertz, A., Le Blay, G., Blaut, M. (2000). Quantification of Different Eubacteriumspp. In Human Fecal Samples With Species-Specific 16s rRNA-Targeted Oligonucleotide Probes. Appl. Environ. Microbiol. 66, 375–382. doi: 10.1128/AEM.66.1.375-382.2000
Shipitsyna, E., Roos, A., Datcu, R., Hallén, A., Fredlund, H., Jensen, J. S., et al. (2013). Composition of the Vaginal Microbiota in Women of Reproductive Age – Sensitive and Specific Molecular Diagnosis of Bacterial Vaginosis Is Possible? PloS One 8, e60670. doi: 10.1371/journal.pone.0060670
Smayevsky, J., Canigia, L. F., Lanza, A., Bianchini, H. (2001). Vaginal Microflora Associated With Bacterial Vaginosis in Nonpregnant Women: Reliability of Sialidase Detection. Infect. Dis. Obstet. Gynecol. 9, 17–22. doi: 10.1155/S1064744901000047
Smith-Dupont, K. B., Wagner, C. E., Witten, J., Conroy, K., Rudoltz, H., Pagidas, K., et al. (2017). Probing the Potential of Mucus Permeability to Signify Preterm Birth Risk. Sci. Rep. 7, 10302–10302. doi: 10.1038/s41598-017-08057-z
Spiegel, C. A. (1991). Bacterial Vaginosis. Clin. Microbiol. Rev. 4, 485–502. doi: 10.1128/CMR.4.4.485
Spiegel, C. A., Roberts, M. (1984). Mobiluncus Gen-Nov, Mobiluncus-Curtisii Subsp Curtisii Sp-Nov, Mobiluncus-Curtisii Subsp Holmesii Subsp-Nov, and Mobiluncus-Mulieris Sp-Nov, Curved Rods From the Human Vagina. Int. J. Syst. Bacteriol. 34, 177–184. doi: 10.1099/00207713-34-2-177
Sprott, M. S., Ingham, H. R., Pattman, R. S., Eisenstadt, R. L., Short, G. R., Narang, H. K., et al. (1983). Characteristics of Motile Curved Rods in Vaginal Secretions. J. Med. Microbiol. 16, 175–182. doi: 10.1099/00222615-16-2-175
Srinivasan, S., Fredricks, D. N. (2008). The Human Vaginal Bacterial Biota and Bacterial Vaginosis. Interdiscip. Perspect. Infect. Dis. 2008, 750479. doi: 10.1155/2008/750479
Srinivasan, S., Hoffman, N. G., Morgan, M. T., Matsen, F. A., Fiedler, T. L., Hall, R. W., et al. (2012). Bacterial Communities in Women With Bacterial Vaginosis: High Resolution Phylogenetic Analyses Reveal Relationships of Microbiota to Clinical Criteria. PloS One 7, e37818. doi: 10.1371/journal.pone.0037818
Srinivasan, S., Morgan, M. T., Fiedler, T. L., Djukovic, D., Hoffman, N. G., Raftery, D., et al. (2015). Metabolic Signatures of Bacterial Vaginosis. MBio 6, e00204–00215. doi: 10.1128/mBio.00204-15
Srinivasan, S., Munch, M. M., Sizova, M. V., Fiedler, T. L., Kohler, C. M., Hoffman, N. G., et al. (2016). More Easily Cultivated Than Identified: Classical Isolation With Molecular Identification of Vaginal Bacteria. J. Infect. Dis. 214 Suppl 1, S21–S28. doi: 10.1093/infdis/jiw192
Sukocheva, O. A., Lukina, E., Mcgowan, E., Bishayee, A. (2020). Sphingolipids as Mediators of Inflammation and Novel Therapeutic Target in Inflammatory Bowel Disease. Adv. Protein Chem. Struct. Biol. 120, 123–158. doi: 10.1016/bs.apcsb.2019.11.003
Teo, C., Kwong, L., Benn, R. (1987). Incidence of Motile, Curved Anaerobic Rods (Mobiluncus Species) in Vaginal Secretions. Pathology 19, 193–196. doi: 10.3109/00313028709077133
Thota, V. R., Dacha, S., Natarajan, A., Nerad, J. (2011). Eggerthella Lenta Bacteremia in a Crohn’s Disease Patient After Ileocecal Resection. Future Microbiol. 6, 595–597. doi: 10.2217/fmb.11.31
Trace.ncbi.nlm.nih.gov (2021) Run Browser : Browse : Sequence Read Archive : NCBI/NLM/NIH. Available at: https://trace.ncbi.nlm.nih.gov/Traces/sra/?run=SRR1518480 (Accessed 24 November 2021).
Vetere, A., Borriello, S. P., Fontaine, E., Reed, P. J., Taylor-Robinson, D. (1987). Characterisation of Anaerobic Curved Rods (Mobiluncus Spp.) Isolated From the Urogenital Tract. J. Med. Microbiol. 23, 279–288. doi: 10.1099/00222615-23-3-279
Ville, Y., Rozenberg, P. (2018). Predictors of Preterm Birth. Best Pract. Res. Clin. Obstet. Gynaecol. 52, 23–32. doi: 10.1016/j.bpobgyn.2018.05.002
Wei, S.-Q., Fraser, W., Luo, Z.-C. (2010). Inflammatory Cytokines and Spontaneous Preterm Birth in Asymptomatic Women: A Systematic Review. Obstet. Gynecol. 116, 393–401. doi: 10.1097/AOG.0b013e3181e6dbc0
Workowski, K. A., Bolan, G. A. (2015). Sexually Transmitted Diseases Treatment Guidelines 2015. MMWR Recomm. Rep. 64, 1–137.
Keywords: vaginal microbiome, vaginal dysbiosis, organotypic 3D culture, biogenic amines (BAs), global metabolic and regulatory networks, women’s health, cervical epithelial barrier, genital inflammation
Citation: McKenzie R, Maarsingh JD, Łaniewski P and Herbst-Kralovetz MM (2021) Immunometabolic Analysis of Mobiluncus mulieris and Eggerthella sp. Reveals Novel Insights Into Their Pathogenic Contributions to the Hallmarks of Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 11:759697. doi: 10.3389/fcimb.2021.759697
Received: 16 August 2021; Accepted: 06 December 2021;
Published: 23 December 2021.
Edited by:
Mariya Ivanova Petrova, KU Leuven, BelgiumReviewed by:
Laura K. Sycuro, University of Calgary, CanadaLiesbeth Demuyser, Flanders Institute for Biotechnology, Belgium
Copyright © 2021 McKenzie, Maarsingh, Łaniewski and Herbst-Kralovetz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melissa M. Herbst-Kralovetz, mherbst1@arizona.edu
 Ross McKenzie
Ross McKenzie Jason D. Maarsingh1
Jason D. Maarsingh1  Paweł Łaniewski
Paweł Łaniewski Melissa M. Herbst-Kralovetz
Melissa M. Herbst-Kralovetz