February 2021—New from CAP Publications is Surgical Pathology Review, by Daniel D. Mais, MD, associate professor of pathology, Department of Pathology, University of Texas Long School of Medicine, San Antonio. He and 14 other contributors wrote this book to ease the transition through board exams and into practice, Dr. Mais writes in the preface. Here is what he told CAP TODAY about the book. (The section on epithelial proliferative lesions in the chapter on breast begins below.)
 You write in your preface that this book is intended to fill a gap. Can you explain what that gap is?
You write in your preface that this book is intended to fill a gap. Can you explain what that gap is?
The available textbooks are of two main types. First, there are some whose purpose is to teach basic concepts in pathology to medical students. The second type is intended to be used by practicing pathologists engaged in diagnostic work. But these two are like circles in a Venn diagram that do not overlap. There is a knowledge set that is beyond the scope of the first type of book and perhaps considered of insufficient practical import for the second. It is this set of knowledge that we thought deserved a textbook.
You also write that some web-based resources are excellent but that in your view a book with a beginning and end is better for the learner in pathology. How so?
The unstated premise is that it is important to have a finite set of facts about which one is certain, to serve as anchors in contextualizing the universe of other facts and conjecture. The bottomlessness of the internet can at times prove very useful in accessing the latter. One of our tasks as educators is to delimit a set of what we consider essential knowledge, as prerequisites for the acquisition of additional knowledge.
How is your book organized and who is your intended audience?
The book is intended for pathologists, at any level of experience but particularly those near the end of training, who seek a once-over review. It is organized in the traditional way, organ by organ. The only exception is the final chapter, titled “Special Topics,” which includes a variety of things applicable throughout. We broke each entity considered into subsections, including its context, clinical findings, prototypical morphologic findings, special studies, and treatment and prognosis.
What can you tell us about the other 14 contributors?
Essential to a project like this is a group of people who share a vision and remain committed until realizing it. In this regard I was grateful to have the help of several colleagues, each having unique subspecialty expertise. Most of them are UT faculty, who provided either original contributions or expert review in ENT pathology (Gabriela Gonzalez), GI pathology (Gonzalez), pancreatic pathology (Gonzalez), liver (Adam Booth), breast (Alia Nazarullah), gynecologic (Philip Valente, Abby Richmond), genitourinary (Richmond), medical renal (Yanli Ding), lung (Sarah Hackman), mediastinum (Hackman, Douglas Warden), skin (Olaoluwa Bode-Omoleye), bone and soft tissue (Josefine Heim-Hall), CNS (Andrea Gilbert), and lymph nodes and spleen (Nazarullah).
Here, from the newly released Surgical Pathology Review, is the section on epithelial proliferative lesions in the chapter on breast. Other sections in that chapter are fibrocystic lesions, benign tumors, papillary lesions, invasive carcinoma, biphasic tumors and stromal tumors, and infectious and inflammatory disorders.
To order (PUB130), call 800-323-4040 option 1 or go to www.cap.org (Shop tab) ($100 for members, $125 for others). For the ebook ($95), go to ebooks.cap.org. If you are interested in writing a book, contact Caryn Tursky at ctursky@cap.org.
Breast: Epithelial proliferative lesions
Usual ductal hyperplasia (UDH)
Context
- UDH is a benign intraductal proliferation of hyperplastic, nonneoplastic, ductal epithelial cells.
- A distinction is often made between mild UDH, which has the risk profile of nonproliferative breast lesions, and moderate to florid UDH, which are associated with a slightly (1.5–2×) increased risk of breast carcinoma.
Clinical findings
- Usually occurs as an incidental finding within fibrocystic changes or other benign lesions.
Prototypical morphology
- UDH is identified when the ductal epithelium exceeds the normal one- to two-cell thickness, after accounting for tangential sectioning and other artifacts.
- Mild UDH is present when the ductal epithelium is up to four cells thick; on the other end of the spectrum, florid UDH fills duct lumens, while moderate UDH is between these extremes.
- The essential features of UDH, in contrast to DCIS and ADH, include cellular heterogeneity and architectural disorganization.
- The hyperplastic cellular population is cytologically heterogeneous and haphazardly arranged, with irregular cell spacing; the effect of this unevenness is luminal structures with rough, wavy borders and elongated, slit-like spaces (Figure 5-7).
- These slit-like spaces are typically found at the periphery of the duct lumen, with intervening epithelial “bridges” that are tapered rather than stout.
- Micropapillary UDH usually has broad-based elongated micropapillae with tapering ends (unlike ADH, which has narrow-based, short, bulbous micropapillae).
- The epithelium may appear spindled and “streaming,” particularly in “bridges” between adjacent spaces where the cells are generally aligned parallel to the lumen and may lay flat against it.
- Individual ductal epithelial cell nuclei are oval and frequently grooved. They have pale dispersed chromatin and small indistinct nucleoli.
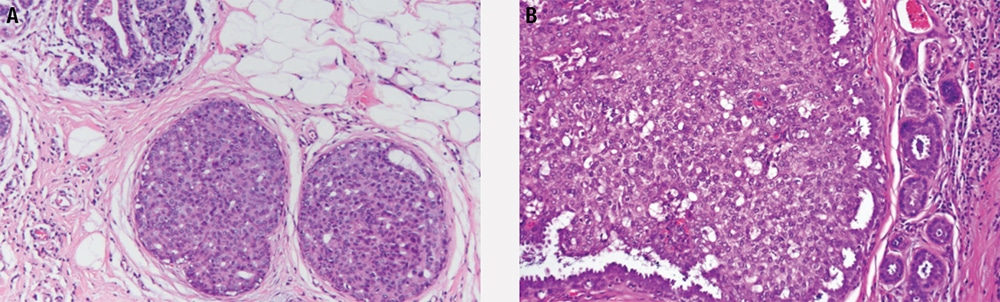
Figure 5-7. Usual ductal hyperplasia. Ducts are expanded (a) with haphazard epithelial cells that form slit-like spaces that are often aligned parallel to the basement membrane along the periphery (b).
Special studies
- CK5 (or other basal keratins) and CK8/18 (luminal keratins) show a mosaic pattern of expression—a reflection of cellular heterogeneity—in contrast to low-grade DCIS and ADH, which are uniformly negative.
- Similarly, ER shows patchy and variable positivity in UDH, in contrast to strong and diffuse expression in ADH and low-grade DCIS.
Treatment and prognosis
- These are benign lesions.
- Aside from mild UDH, there is a 1.5–2× risk for subsequent breast cancer (similar to other proliferative lesions without atypia).
Flat epithelial atypia (FEA)
Context
- Nonobligate precursor lesion, clonally related to low-grade DCIS and tubular carcinoma, a relationship manifested in Rosen’s triad (association of flat epithelial atypia, lobular neoplasia, and tubular carcinoma).
Clinical findings
- FEA often presents as an incidental finding or as mammographic microcalcifications.
Prototypical morphology
- There are expanded terminal duct-lobular units in a pattern similar to blunt duct adenosis/columnar cell alteration; however, the luminal spaces tend to be more rigid and round.
- As in blunt duct adenosis/columnar cell alteration, the cells may be columnar or cuboidal, and apical cytoplasmic snouts and luminal calcifications are often present.
- The luminal cells (Figure 5-8) are monomorphic, contain small nucleoli, and have nuclei that are enlarged, rounded, and without polarity; these are the main features that distinguish FEA from blunt duct adenosis/columnar cell alteration, and these are the features that it has in common with ADH/DCIS.
- The epithelial proliferation is flat (it may be pseudostratified but is not stratified), with no architectural complexity or bridging, in contrast to ADH/DCIS.
- Careful evaluation of adjacent foci for atypical lobular hyperplasia, ADH, and DCIS is recommended.
Special studies - The luminal cells are strongly positive for ER and Bcl-2.
Treatment and prognosis
- Excision is not recommended where there is good radiologic/pathologic correlation.
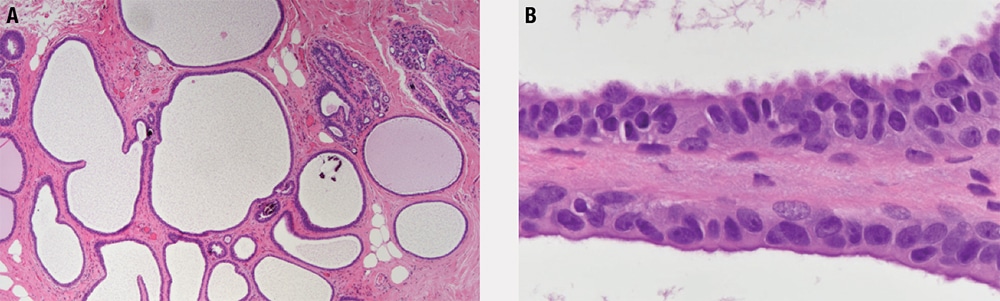
Figure 5-8. Flat epithelial atypia. Tubules have profiles similar to those seen in blunt duct adenosis (a) and have a flat inner lining of cells with nuclear enlargement, loss of polarity, and prominent nucleoli (b).
Atypical ductal hyperplasia (ADH)
Context
- Nonobligate precursor lesion, clonally related to low-grade DCIS.
Clinical findings
- ADH presents most often as clustered microcalcifications.

Figure 5-9. Atypical ductal hyperplasia. The features of low-grade ductal carcinoma in situ are present within a single duct profile.
Prototypical morphology
- ADH is an intraductal proliferation (Figure 5-9) in which the qualitative morphologic features of low-grade DCIS are present focally.
- Ducts are mildly expanded, and there is architectural complexity with punched-out cribriform spaces, micropapillae, or rigid arched bridges.
- The cells are low grade and monomorphic with round nuclei, condensed chromatin and inconspicuous nucleoli (intermediate or high nuclear grade, regardless of quantitative extent, are characteristics of DCIS).
- The atypical proliferation involves less than 2 mm extent or less than two contiguous ducts (greater than 2 mm or two contiguous ducts characterized as DCIS).
Special studies
- CK5/6 is negative in the intraductal population (unlike mosaic positivity in UDH).
- ER positivity is strong and diffuse (unlike variable positivity in UDH).
Treatment and prognosis
- Excision is recommended to rule out DCIS or invasive carcinoma.
- ADH confers 4–5× increase in relative risk for subsequent breast cancer.
Atypical lobular hyperplasia (ALH)
Context
- Nonobligate precursor lesion associated with bilateral increased risk of breast cancer.
- ALH is cytologically similar to lobular carcinoma in situ but is limited in extent.
- Together, ALH and lobular carcinoma in situ (LCIS) are referred to as lobular neoplasia.
- Lobular neoplasia tends to be multicentric and bilateral.
Clinical findings
- These are asymptomatic, incidental lesions.

Figure 5-10. Atypical lobular hyperplasia. The features of lobular carcinoma in situ are present within a minority of acini of a lobular unit (a) and demonstrate loss of E-cadherin (b).
Prototypical morphology
- Distended lobules (Figure 5-10) are seen filled with loosely cohesive, monomorphic cells with low-grade nuclei.
- ALH involves less than 50% of acini in a terminal ductal lobular unit (more than this constitutes LCIS).
Special studies
- Absence of membranous E-cadherin staining (in contrast to ductal proliferations).
- Absence of nuclear beta catenin (in contrast to ductal proliferations).
- Cytoplasmic p120 staining (as opposed to membranous staining in ductal lesions).
Treatment and prognosis
- Treatment options include surveillance, chemoprevention with tamoxifen, and excision.
- ALH confers 4–5× increase in relative risk for subsequent breast cancer (including both ductal and lobular types).
Lobular carcinoma in situ (LCIS)
Context
- LCIS, together with ALH, is considered part of the lobular neoplasia spectrum.
- There is poor reproducibility in the distinction of ALH and LCIS.
- LCIS is frequently associated with flat epithelial atypia.
- LCIS is the most common in situ lesion in fibroadenoma.
- LCIS is multicentric and/or bilateral in 50% of cases.
Clinical findings
- Usually an incidental finding, although up to 15% of cases associated with microcalcifications.
- Pleomorphic/florid LCIS may present as a mass and is more likely to have calcifications.
Prototypical morphology
- The lobule is expanded (Figure 5-11), and acini are filled with small, loosely cohesive monomorphic cells (classic type), by definition in greater than 50% of a terminal duct lobular unit.
- When involving ducts, the neoplastic cells proliferate between basement membrane and luminal epithelial cells in a clover leaf-like pattern.
- Pleomorphic LCIS shows increased nuclear pleomorphism and atypia, sometimes with central necrosis and calcification, closely resembling DCIS.
- Florid LCIS shows classic nuclear features but fills and distends ducts in a DCIS-like pattern, often with comedonecrosis.

Figure 5-11. Lobular carcinoma in situ. The entire lobular unit is expanded by a monotonous population of small round noncohesive cells.
Special studies
- Negative for membranous E-cadherin staining (in contrast to ductal lesions).
- Negative for nuclear beta catenin (in contrast to ductal lesions).
- Cytoplasmic p120 staining (membranous staining in ductal lesions).
Treatment and prognosis
- LCIS confers 8 –10× risk of malignancy for bilateral breasts.
- Management may include follow-up, tamoxifen, or excision.
- Excision with margin clearance is recommended for pleomorphic LCIS.
Ductal carcinoma in situ (DCIS)
Context
- Clonal proliferation of epithelial cells surrounded by myoepithelial cell layer and confined within the basement membrane.
- High-grade DCIS and low-grade DCIS appear to be distinct processes at the molecular level, with intermediate-grade DCIS representing a mixture of these two types.
Clinical findings
- High-grade DCIS can be mass forming, and some cases present as nipple discharge, but over 90% of cases are detected by mammography.
- Radiographically, DCIS usually presents as microcalcifications. Those associated with low-grade DCIS are often described as clustered, fine, and crystalline, while in high-grade DCIS, they are described as large, amorphous, linear, and branching.
Prototypical morphology
- Grossly, most cases demonstrate no apparent lesion. There may be cheesy material identified within dilated spaces in comedo-type DCIS, and in other cases there may be an indurated lesion.
- DCIS can be low, intermediate, or high grade (Figure 5-12). Grading is based largely upon nuclear features, but some grading systems take necrosis into account.
- Architectural patterns include solid, cribriform (with rigid punched-out spaces), comedo, papillary, micropapillary, clinging, etc, but these are often mixed, and reproducibility is poor.
- Necrosis must be distinguished from luminal secretion, which lacks evidence of cell death and instead consists solely of acellular eosinophilic granular material. Sometimes necrosis occurs in the center of a duct involved by solid-type DCIS, and this is called comedonecrosis. In other cases, central necrosis is present with cribriform or other patterns, while in still other cases necrosis may involve random individual cells (“punctate” necrosis).
- Low-grade DCIS is by definition greater than 2 mm or involves more than two contiguous ducts, while there is not a quantitative criterion attached to high-grade DCIS.
- Extensive high-grade DCIS may be associated with foci of microinvasion.

Figure 5-12. Ductal carcinoma in situ (DCIS). Examples of DCIS including intermediate-grade cribriform (a), micropapillary (b), and high-grade cribriform with central necrosis (c).
Special studies
- Low-grade DCIS usually shows strong and diffuse ER expression. CK5/6, human epidermal growth factor receptor 2 (HER2), and p53 are negative.
- High-grade DCIS can show variable ER and CK5/6 expression and often overexpresses HER2 and p53.
Treatment and prognosis
- High-grade DCIS more likely to recur and to progress than low-grade DCIS.
- For all grades, local excision is recommended, with partial or total mastectomy.
- Sentinel lymph node biopsy may be considered for extensive high-grade DCIS, and in such cases lymph node metastases are occasionally encountered.
- Margin status is the strongest predictor of local recurrence. It is known that the risk of recurrence is lower with greater distance from nearest margin, but there is not a universally accepted threshold distance.
- Tamoxifen therapy is known to reduce the risk of local recurrence.
- DCIS confers 8 –10× risk of malignancy in ipsilateral breast.
Paget disease of nipple
Context
- Paget disease is the presence of breast carcinoma within the nipple epidermis.
- The majority of lesions are associated with underlying DCIS or invasive carcinoma.
Clinical findings
- The cutaneous changes resemble eczema: crusted erythematous lesions with scale.
Prototypical morphology
- Large cells with abundant pale cytoplasm are present within the epidermis, arranged singly (“buckshot”) or in small groups (Figure 5-13).
- The cells have atypical nuclear features and intracytoplasmic mucin.
- A lichenoid dermal infiltrate may be seen.

Figure 5-13. Paget disease of the nipple.
Special studies
- Paget cells are consistently positive for CK7 and mucin stains.
- They are variably positive for carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), GCDFP, and GATA3.
- HER2 is overexpressed in most cases, and ER/PR are usually negative.
- Paget cells are negative for high-molecular-weight keratins, p63, and melanocytic markers.
- The immunophenotype of Paget cells may be indistinguishable from benign Toker cells; they are distinguished based on cytologic atypia.
Treatment and prognosis
- Prognosis and treatment depend on presence of underlying invasive carcinoma.
- Pure Paget disease, without underlying malignant breast lesion, has excellent overall survival, with lymph node metastases being rare.