Normal Physiology of the Urogenital System
The normal microbiota of the urogenital system provides critical nonspecific protection against infectious diseases. In males, the normal microbiota is comprised of bacterial species related to skin microbiota and is located within the distal urethra. In females, the normal microbiota is located within the vagina and the distal one-third of the urethra. Within the vagina, normally there is a population of bacteria called Lactobacillus acidophilus, which assist in maintaining acidic pH levels. Most pathogenic bacteria are not as likely to thrive in acidic environments; so this acidic pH, in combination with a thicker squamous epithelium of the vagina, serves as a protection against potential infections (McCance & Huether, 2019).
Pathophysiology of Chlamydia
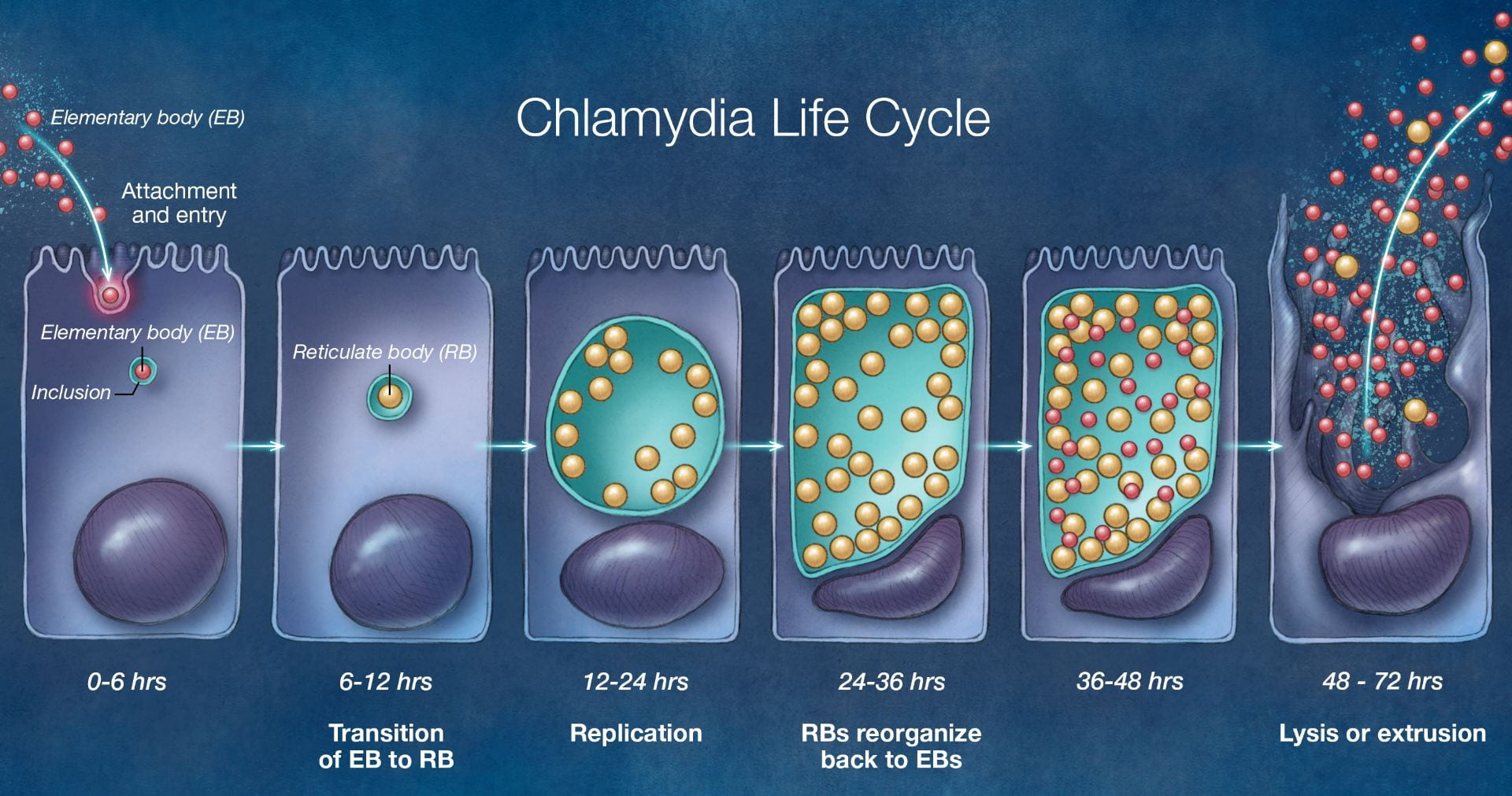
Figure 6: The life cycle of the pathogenic C. trachomatis bacteria.
Image taken from https://www.std.uw.edu/go/pathogen-based/chlamydia/core-concept/all
The cases of sexually transmitted diseases are at an all-time high right now. There are 1.8 million cases of chlamydia in the United States, which is a 19% increase since 2014 (LaChance, 2019). It is the most commonly reported bacterial infection in the United States and the most common sexually transmitted disease worldwide. It is caused by Chlamydia trachomatis bacteria which infects both men and women. Chlamydia trachomatis are gram-negative anaerobic bacteria that replicate inside eukaryotic cells (Mohseni, 2019). It is a weak organism that relies on its host for nutrients and survival. It lives inside a host in order to reproduce and survive. C. trachomatis targets squamocolumnar epithelial cells of the endocervix and upper genital tract in women, and the conjunctiva, urethra, and rectum in both men and women (Mohseni, 2019). Chlamydia has two developmental forms, elementary bodies (EB) and reticulate bodies (RB). Elementary bodies are the infectious version of C. trachomatis and reticulate bodies are the growth version of C. trachomatis (“Pathophysiology of chlamydia,” n.d.). Elementary bodies are metabolically inactive and enter the host cell through endocytosis. Once it enters the host cell, it forms into metabolically active reticulate bodies. Reticulate bodies then start using the nutrients of the host cell and start to reproduce and form multiple reticulate bodies through binary fission. RBs then start forming EBs in order to infect more cells. This excess production of EBs causes the cell to rupture and die. EBs and RBs get released into the extracellular matrix and more host cells come and try to eat these bodies and the cycle continues (“Pathophysiology of chlamydia,” n.d.).

Figure 7: Cervicitis and vaginal discharge as a result of a C. trachomatis infection.
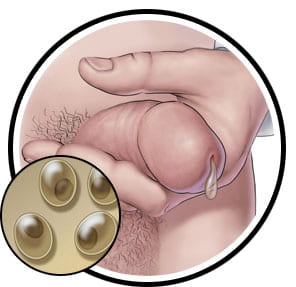
Figure 7: Penile discharge caused by infection of C. trachomatis.
Images taken from https://www.sexandu.ca/stis/chlamydia/
There are 18 serologically variant serovars of Chlamydia trachomatis. Serovars A, B, Ba, C are associated with chronic conjunctivitis, which can lead to blindness. Serovars L1-L3 cause Lymphogranuloma venereum, which begins as a skin lesion and spreads to the lymphatic system and causes infection. Serovars D-K are associated with genital tract infection (Mohseni, 2019). The disease can be transmitted through oral, vaginal, or anal sex with someone who has chlamydia. It can also be spread vertically from a mother to their children during delivery (“STD Facts – Chlamydia,” 2014). The prevalence rate is higher in women than in men. It is twice as prevalent in women as men. It is more prevalent in females ages 15-24 and men ages 20-24 (Mohseni, 2019). Infection rates are approximately 6 times higher in African Americans than in Caucasians. Some of the risk factors of chlamydia include number of lifetime sexual partners, no use of barrier contraception, young females, Black/Hispanic/Native American and Alaskan Native ethnicity, and homosexuality (Cashman, 2019). Chlamydia is usually asymptomatic in most people, but if left untreated, it may cause multiple clinical syndromes such as genital infection, cervicitis, urethritis, pelvic inflammatory disease. The cervix is the most common site of genital infection in women and the infection can ascend and cause pelvic inflammatory disease. These syndromes can cause symptoms such as vaginal discharge, intermenstrual bleeding, dysuria, pyuria, tubal infertility, ectopic pregnancy, and chronic pelvic pain (Hsu, n.d.). Most men rarely have health problems due to chlamydia however, some men can show symptoms such as discharge from the penis, burning sensation during urination, and pain and swelling in the testicles. Untreated chlamydia can also increase the chance of giving or getting HIV (“STD Facts – Chlamydia,” 2014).
The CDC recommends regular screening for general prevention. Endocervical NAAT or urine tests are some of the recommended screening tests (Mohseni, 2019). Screening is recommended for anyone who has had a new partner or more than one sex partner in the past 6 months. All sexually active men and women 25 and below are recommended to be screened at least once a year (“Chlamydia Infections,” 2019). Screening for pregnant women and women above 25 years is also recommended if identifiable risk factors are present. Chlamydia trachomatis can be treated with antibiotic treatment and the prognosis rate is 95% if initiated early and the full course is completed (Mohseni, 2019).
Laboratory Diagnosis-
- NAAT- Nucleic acid amplification test detects genetic material that is specific for the organism. It can detect both living or non-viable organisms. Urine specimens, vaginal swabs, urethral swabs, endocervical swabs, and rectal swabs can be used to collect samples for testing (Hanh & Geisler, 2018). Sensitivity > 95%, specificity > 99% (Cashman, 2019).
- Culture- Columnar cells are collected in order to detect for C. trachomatis. Lower sensitivity than NAAT, approximately 50% (Hanh & Geisler, 2018).