- Department of Neurological Surgery, University of California, UCI Medical Center, Orange, United States.
- Department of Neurosurgery, UC San Diego School of Medicine, La Jolla, United States.
- Department of Pathology, University of California, UCI Medical Center, Orange, California, United States.
- Department of Orthopedic Surgery, University of California, UCI Medical Center, Orange, California, United States.
Correspondence Address:
Frank P. K. Hsu
Department of Neurological Surgery, University of California, UCI Medical Center, Orange, United States.
DOI:10.25259/SNI_768_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Brian V. Lien1, Nolan J. Brown1, Alexander S. Himstead1, Benjamin Z. Ball1, Aileen Guillen1, Nischal Acharya1, Chen Yi Yang1, Ronald Sahyouni2, Mari Perez-Rosendahl3, Russell N. Stitzlein4, Frank P. K. Hsu1. Surgical management of a rare myxopapillary ependymoma of the gluteal region: A case report. 30-Mar-2021;12:130
How to cite this URL: Brian V. Lien1, Nolan J. Brown1, Alexander S. Himstead1, Benjamin Z. Ball1, Aileen Guillen1, Nischal Acharya1, Chen Yi Yang1, Ronald Sahyouni2, Mari Perez-Rosendahl3, Russell N. Stitzlein4, Frank P. K. Hsu1. Surgical management of a rare myxopapillary ependymoma of the gluteal region: A case report. 30-Mar-2021;12:130. Available from: https://surgicalneurologyint.com/surgicalint-articles/10679/
Abstract
Background: Ependymomas are rare tumors originating from neuroepithelial cells lining the wall of the ventricles or central canal of the spinal cord. While these tumors mainly occur within the central nervous system (CNS), there are occasional reports in children and young adult patients with a primary tumor occurrence outside of the CNS. Ependymomas of the sacrococcygeal region have been infrequently described in the literature with no standard of care established. We present a case report and review of the literature regarding this rare entity.
Case Description: A 24-year-old woman presented with right gluteal pain worsened by sitting and a palpable soft tissue mass of the sacrococcygeal region. Magnetic resonance imaging revealed a 3.7 cm cystic mass centered in the right gluteal region. She underwent a biopsy at an outside institution, with histology revealing myxopapillary ependymoma. The patient was referred to our hospital and underwent an interdisciplinary neurosurgical and orthopedic oncology en bloc resection of the ependymoma, which intraoperatively appeared to originate from the coccygeal nerve.
Conclusion: In the present report, the authors demonstrate that a myxopapillary ependymoma may present as an isolated gluteal mass attached to the coccygeal nerve, without frank CNS involvement. Furthermore, an interdisciplinary approach to surgical resection of this lesion appears to represent an effective treatment modality.
Keywords: Extra central nervous system ependymoma, Extraneural ependymoma, Gluteal ependymoma, Rare ependymoma case, Rare myxopapillary ependymoma
INTRODUCTION
Ependymomas occurring outside of the central nervous system (CNS), otherwise known as extra CNS ependymomas, are extremely uncommon and only occasionally described in the literature, with most reports featuring only one to two cases per institution.[
METHODS
In January 2021, we performed a systematic review of three literature databases to identify previous reports of gluteal myxopapillary ependymoma. No time restriction was applied to allow for a comprehensive search. The following search terms were used: (gluteal myxopapillary ependymoma) OR (sacrococcygeal myxopapillary ependymoma) OR (coccygeal myxopapillary ependymoma). There were a total of 302 publications: 131 from PubMed, 87 results from Scopus, and 84 results from Web of Science. There were 211 publications after deletion of duplicates. We included all cases of primary myxopapillary ependymoma presenting as a gluteal mass confirmed to have no association with the CNS. Studies were excluded if they were not in English or not journal articles (e.g., conference abstracts).
CASE REPORT
A 24-year-old, otherwise healthy female with a strong family history of cancer (including glioma) presented with gradually worsening right gluteal pain exacerbated by sitting and an enlarging gluteal mass. She denied any back pain, leg pain, focal motor weakness, paresthesias, or bowel/bladder dysfunction. Physical exam revealed no focal neurological deficits. Upon inspection of the buttocks, a mass was observable on the right intergluteal cleft and tender to palpation. The patient underwent a biopsy at an outside institution, which revealed myxopapillary ependymoma, before being referred to our hospital.
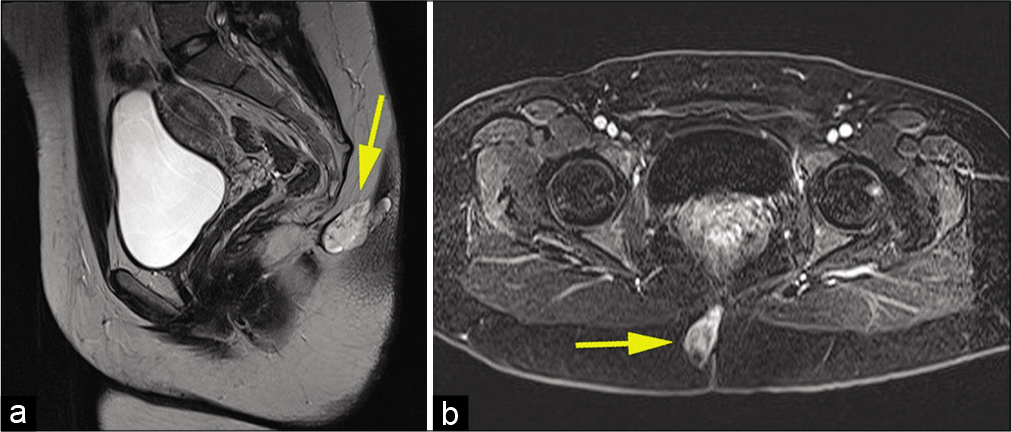
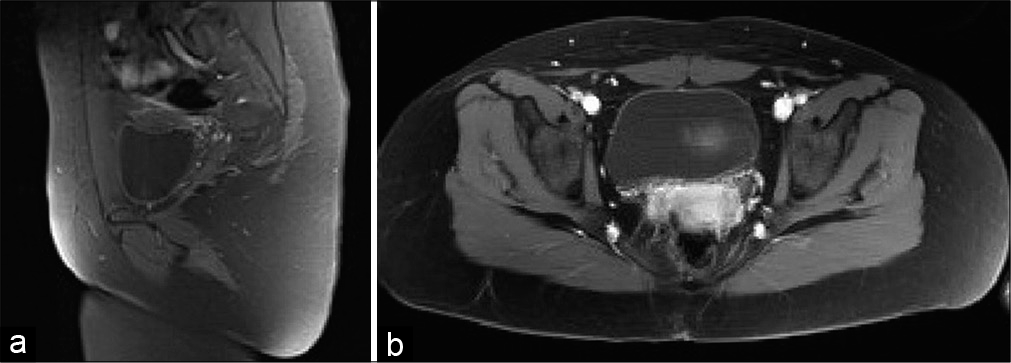
Work-up proceeded with full neuro-axis imaging including magnetic resonance imaging (MRI) of the brain and MRI/ computed tomography myelograms of the cervical, thoracic, and lumbar spine, which were all unremarkable. Pelvic MRI demonstrated a T2 hyperintense cystic lesion with enhancing septa in the right paramedian gluteal region that measured 3.7 ×1.8 ×3.3 cm. The lesion extended from the posterior cortex of the second coccygeal segment body to the right gluteal cleft skin. There was no evidence of any intrinsic spinal cord component or definite CSF connection to the central canal [
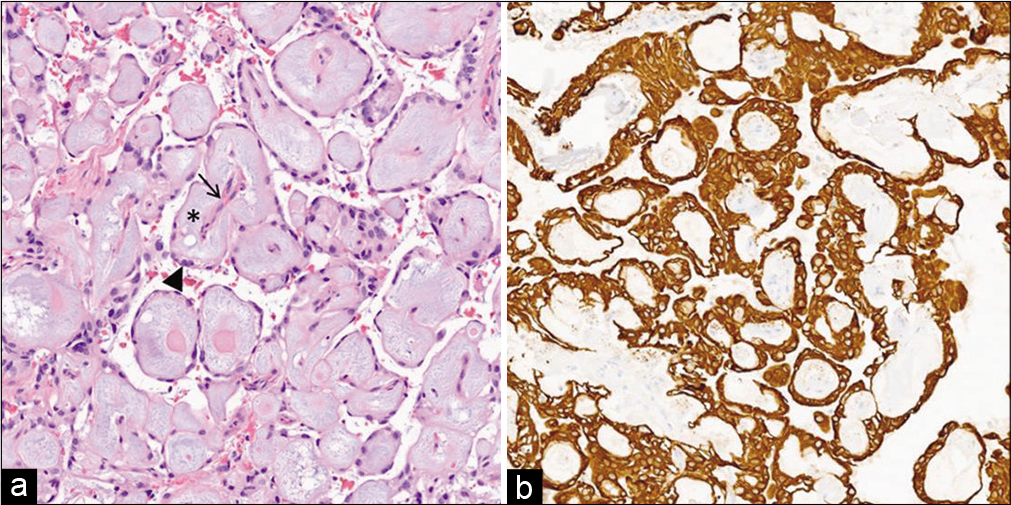
An interdisciplinary neurosurgical and orthopedic oncology en bloc resection was planned. Intraoperatively, it was noted that the lesion was attached to the left coccygeal nerve. A 2.0 silk tie was secured around the base of the left coccygeal nerve, and it was coagulated and sharply divided ~5 mm proximal to the silk tie. Kerrison rongeurs were used to remove a portion of the coccyx which appeared to be infiltrated with tumor. Intraoperative frozen pathology was consistent with myxopapillary ependymoma with negative margins [
DISCUSSION
Ependymomas are distinct histopathological tumors of the CNS that affects both children and adults.[
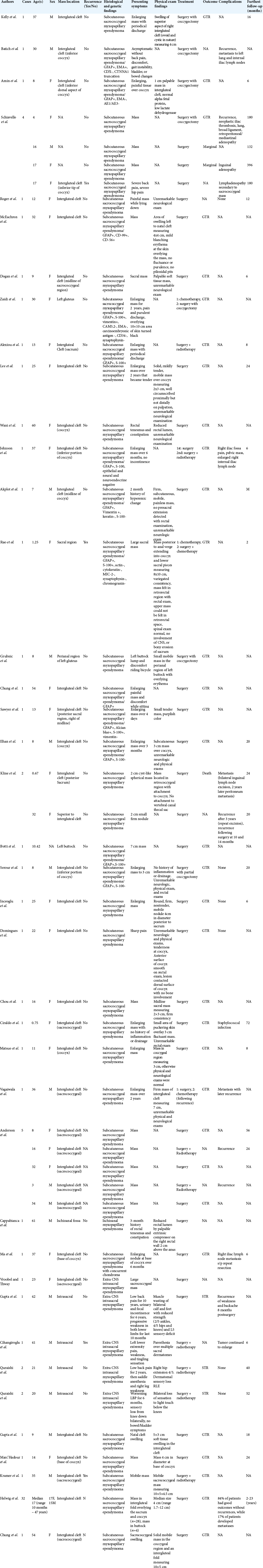
In our literature review, we found 38 studies that described 78 unique cases of myxopapillary ependymoma occurring in the sacrococcygeal region without extension into the CNS [
As demonstrated by our literature review, the exact presentation of sacrococcygeal ependymomas is variable. Given their location and propensity for drainage, they are frequently misdiagnosed as the more frequently encountered pilonidal cyst.[
We identified 13 cases of recurrence or metastasis out of 78 total cases (16.7%) in the literature. Notably, this number may be artificially low as recurrence typically occurs up to 20 years after initial resection, and several studies reported follow-up of 6 months or less.[
The primary means of management for sacrococcygeal myxopapillary ependymoma is complete surgical resection.[
Management of subcutaneous sacrococcygeal myxopapillary ependymoma is multifaceted and not fully standardized due to rarity of the disease. Our case is unique in that it describes a myxopapillary ependymoma presenting as palpable gluteal mass adherent to the coccygeal nerve and was resected successfully en bloc with a multidisciplinary team. While palpable mass is the most common presenting complaint of this entity, we did not find any reports of extra CNS ependymomas attached to the coccygeal nerve. As the tumor did partially invade the coccyx, partial coccygectomy was performed in order to attain en bloc gross-total resection. No local lymph nodes were involved, and no distant metastases were detected on imaging, so radiotherapy was deferred for the time being. The patient remains without recurrence at 6 months and will continue with long-term surveillance.
Management of subcutaneous sacrococcygeal myxopapillary ependymoma is multifaceted and not fully standardized due to rarity of the disease, and we propose that early intervention by a multidisciplinary team involving neurosurgery, orthopedic surgery, and radiation oncology and long-term radiographic surveillance for disease recurrence is a reasonable treatment strategy.
CONCLUSION
We report a rare case of an extra CNS myxopapillary ependymoma in the gluteal region with attachment to the coccygeal nerve. Management of subcutaneous sacrococcygeal myxopapillary ependymoma is multifaceted and not fully standardized due to the rarity of the disease. We propose that early intervention by a multidisciplinary team involving neurosurgery, orthopedic surgery, and radiation oncology, along with long-term radiographic surveillance for disease recurrence is a reasonable treatment strategy.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akpolat N, Bozlak N, Kazez A, Köseoğullari AA. Sacrococcygeal extraspinal ependymoma: A case report. Turk J Pediatr. 2003. 45: 276-9
2. Aktuğ T, Hakgüder G, Sarioğlu S, Akgür FM, Olguner M, Pabuçcuoğlu U. Sacrococcygeal extraspinal ependymomas: The role of coccygectomy. J Pediatr Surg. 2000. 35: 515-8
3. Alexiou GA, Sfakianos G, Moschovi M, Athanasiadou S, Stefanaki K, Prodromou N. Myxopapillary ependymoma of the sacrococcygeal region presenting as a pilonidal sinus. Pediatr Neurosurg. 2012. 48: 64-65
4. Amin R, Berdan E, Knipstein J, Jarzembowski J, Siddiqui S. Extraspinal sacrococcygeal ependymoma masquerading as sacrococcygeal teratoma in the pediatric patient. Pediatr Surg Int. 2018. 34: 109-12
5. Anderson MS. Myxopapillary ependymomas presenting in the soft tissue over the sacrococcygeal region. Cancer. 1966. 19: 585-90
6. Batich KA, Riedel RF, Kirkpatrick JP, Tong BC, Eward WC, Tan CL. Recurrent extradural myxopapillary ependymoma with oligometastatic spread. Front Oncol. 2019. 9: 1322
7. Botti G, Gravina A, Cremona F, Izzo F, Rigutini M, Picone A. Subcutaneous sacrococcygeal myxopapillary ependymoma: A case report. Eur J Cancer. 1994. 30a: 570-1
8. Cappabianca S, Barberi A, Grassi R, Lieto E, Fulciniti F, Galizia G. Myxopapillary ependymoma of the ischioanal fossa. Br J Radiol. 2003. 76: 659-61
9. Chou S, Soucy P, Carpenter B. Extraspinal ependymoma. J Pediatr Surg. 1987. 22: 802-3
10. Chung JY, Lee SK, Yang KH, Song MK. Subcutaneous sacrococcygeal myxopapillary ependymoma. AJNR Am J Neuroradiol. 1999. 20: 344-6
11. Cihangiroglu M, Hartker FW, Lee M, Sehgal V, Ramsey RG. Intraosseous sacral myxopapillary ependymoma and the differential diagnosis of sacral tumors. J Neuroimaging. 2001. 11: 330-2
12. Ciraldo AV, Platt MS, Agamanolis DP, Boeckman CR. Sacrococcygeal myxopapillary ependymomas and ependymal rests in infants and children. J Pediatr Surg. 1986. 21: 49-52
13. Dogan MS, Collins R, Wang J, Koral K. Subcutaneous sacrococcygeal ependymoma in a child. Spine J. 2016. 16: e513-4
14. Domingues R, Mikulis D, Swearingen B, Tompkins R, Rosen B. Subcutaneous sacrococcygeal myxopapillary ependymoma: CT and MR findings. Am J Neuroradiol. 1991. 12: 171-2
15. Fukuoka K, Kanemura Y, Shofuda T, Fukushima S, Yamashita S, Narushima D. Significance of molecular classification of ependymomas: C11orf95-RELA fusion-negative supratentorial ependymomas are a heterogeneous group of tumors. Acta Neuropathol Commun. 2018. 6: 134
16. Gerstner ER, Pajtler KW. Ependymoma. Semin Neurol. 2018. 38: 104-11
17. Grubnic S, MacVicar D. A perianal problem. Br J Radiol. 1999. 72: 1235-6
18. Gupta R, Rishi A, Suri V, Sharma MC, Gupta A, Garg A. Sacral myxopapillary ependymoma with extensive osteolysis. J Neurooncol. 2008. 86: 349-52
19. Gupta T, Patel V, El-Medani F, Gupta S. An unexpected diagnosis of paediatric subcutaneous sacrococcygeal extraspinal ependymoma: Lessons learnt and review of the literature. J Surg Case Rep. 2020. 2020: rjaa177
20. Helwig EB, Stern JB. Subcutaneous sacrococcygeal myxopapillary ependymoma. A clinicopathologic study of 32 cases. Am J Clin Pathol. 1984. 81: 156-61
21. Ilhan I, Berberoglu S, Kutluay L, Maden HA. Subcutaneous sacrococcygeal myxopapillary ependymoma. Med Pediatr Oncol. 1998. 30: 81-4
22. Inceoğlu R, Ozer F, Pamir N, Küllü S. Extraspinal ependymoma presenting as a subcutaneous mass posterior to the sacrococcygeal region: Case report. Paraplegia. 1993. 31: 800-2
23. Johnson JM, Jessurun J, Leonard A. Sacrococcygeal ependymoma: Case report and review of the literature. J Pediatr Surg. 1999. 34: 1405-7
24. Kelly A, Nally D, Crowther S, Kavanagh D. Subcutaneous sacrococcygeal myxopapillary ependymoma misdiagnosed as pilonidal disease. BMJ Case Rep. 2020. 13: e231639
25. Kline MJ, Kays DW, Rojiani AM. Extradural myxopapillary ependymoma: Report of two cases and review of the literature. Pediatr Pathol Lab Med. 1996. 16: 813-22
26. Kramer GW, Rutten E, Sloof J. Subcutaneous sacrococcygeal ependymoma with inguinal lymph node metastasis: Case report. J Neurosurg. 1988. 68: 474-7
27. Kumar P, Rastogi N, Jain M, Chhabra P. Extraneural metastases in anaplastic ependymoma. J Cancer Res Ther. 2007. 3: 102-4
28. Lee KJ, Min BW, Seo HJ, Cho CH. Subcutaneous sacrococcygeal myxopapillary ependymoma in asian female: A case report. J Clin Med Res. 2012. 4: 61-3
29. Lemberger A, Stein M, Doron J, Fried G, Goldsher D, Feinsod M. Sacrococcygeal extradural ependymoma. Cancer. 1989. 64: 1156-9
30. Lynch J, Kelly N, Fitzpatrick B, Regan P. A sacrococcygeal extraspinal ependymoma in a 67-year-old man: A case report and review of the literature. Br J Plast Surg. 2002. 55: 80-2
31. Ma YT, Ramachandra P, Spooner D. Case report: Primary subcutaneous sacrococcygeal ependymoma: A case report and review of the literature. Br J Radiol. 2006. 79: 445-7
32. Mack SC, Pajtler KW, Chavez L, Okonechnikov K, Bertrand KC, Wang X. Therapeutic targeting of ependymoma as informed by oncogenic enhancer profiling. Nature. 2018. 553: 101-5
33. Marc’Hadour FL, Pasquier B. Subcutaneous sacrococcygeal ependymoma with incidental glomus coccygeum. Histopathology. 1991. 18: 570-2
34. Matsuo K, Kumagai K, Kawai K, Tsuchiyama H. Subcutaneous sacrococcygeal myxopapillary ependymoma: A case report and review of the literatures. Pathol Int. 1985. 35: 925-31
35. McEachron KR, Gaertner WB. Extradural sacrococcygeal subcutaneous ependymoma misdiagnosed as pilonidal disease: Case report and review of the literature. J Surg Case Rep. 2016. 2016: 261369
36. Morantz RA, Kepes JJ, Batnitzky S, Masterson BJ. Extraspinal ependymomas. Report of three cases. J Neurosurg. 1979. 51: 383-91
37. Pajtler KW, Mack SC, Ramaswamy V, Smith CA, Witt H, Smith A. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017. 133: 5-12
38. Quraishi NA, Wolinsky JP, Bydon A, Witham T, Gokaslan ZL. Giant destructive myxopapillary ependymomas of the sacrum. J Neurosurg Spine. 2010. 12: 154-9
39. Rao IS, Kapila K, Aggarwal S, Ray R, Gupta AK, Verma K. Subcutaneous myxopapillary ependymoma presenting as a childhood sacrococcygeal tumor: A case report. Diagn Cytopathol. 2002. 27: 303-7
40. Rogers S, Jones DT, Ireland A, Gottardo NG, Endersby R. Unusual paediatric spinal myxopapillary ependymomas: Unique molecular entities or pathological variations on a theme?. J Clin Neurosci. 2018. 50: 144-8
41. Sawyer JR, Miller JP, Ellison DA. Clonal telomeric fusions and chromosome instability in a subcutaneous sacrococcygeal myxopapillary ependymoma. Cancer Genet Cytogenet. 1998. 100: 169-75
42. Schiavello E, Biassoni V, Antonelli M, Modena P, Cesaro S, Pierani P. Pediatric extraspinal sacrococcygeal ependymoma (ESE): An Italian AIEOP experience of six cases and literature review. Childs Nerv Syst. 2018. 34: 1291-8
43. Serour F, Gorenstein A, Finci Y, Zaidel L. Subcutaneous sacrococcygeal myxopapillary ependymoma. Pediatr Surg Int. 1993. 8: 362-5
44. Sonneland PR, Scheithauer BW, Onofrio BM. Myxopapillary ependymoma. A clinicopathologic and immunocytochemical study of 77 cases. Cancer. 1985. 56: 883-93
45. Vagaiwala MR, Robinson JS, Galicich JH, Gralla RJ, Helson L, Beattie EJ. Metastasizing extradural ependymoma of the sacrococcygeal region: Case report and review of literature. Cancer. 1979. 44: 326-33
46. Vega-Orozco R, Rembao-Bojórquez D, Salmerón-Mercado M, García-Marquez A, Tena-Suck ML. Inguinal lymph nodal metastasis of myxopapillary ependymoma confirmed by fine-needle aspiration cytology, biopsy, and immunohistochemistry: Case report. Diagn Cytopathol. 2011. 39: 689-93
47. Vroobel K, Thway K. Synchronous sacrococcygeal myxopapillary ependymoma and chordoma. Int J Surg Pathol. 2016. 24: 48-50
48. Wani NA, Mir F, Qayum A, Nazir P. Sacrococcygeal extraspinal ependymoma. Acta Neurochir (Wien). 2010. 152: 917-8
49. Yust Katz S, Cachia D, Kamiya-Matsuoka C, Olar A, Theeler B, Prado MP. Ependymomas arising outside of the central nervous system: A case series and literature review. J Clin Neurosci. 2018. 47: 202-7
50. Zaidi SN, Al-Khalidi H, Al-Rikabi AC. Myxopapillary ependymoma masquerading as subcutaneous sacrococcygeal non-healing ulcer: Case report. Ann Saudi Med. 2014. 34: 262-4