- Department of Neurosurgery, Dell Medical School, Austin,
- Jan and Dan Duncan Neurological Research Institute, Texas Children’s Hospital,
- Department of Neurosurgery, Baylor College of Medicine, Houston,
- Department of Neurosurgery, University of Texas Southwestern, Dallas,
- Department of Pathology, Baylor College of Medicine,
- Department of Otolaryngology-Head and Neck Surgery, Baylor College of Medicine, Houston, Texas, United States.
Correspondence Address:
Akash J. Patel, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, United States.
DOI:10.25259/SNI_513_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Darsh S. Shah1,2,3, Himanshu Sharma3, Prem Patel4, Arya Shetty3, Collin William English2,3, J. Clay Goodman5, Ashwin Viswanathan3, Akash J. Patel2,3,6. Recurrent liponeurocytoma: A case report and systematic review of the literature. 02-Sep-2022;13:395
How to cite this URL: Darsh S. Shah1,2,3, Himanshu Sharma3, Prem Patel4, Arya Shetty3, Collin William English2,3, J. Clay Goodman5, Ashwin Viswanathan3, Akash J. Patel2,3,6. Recurrent liponeurocytoma: A case report and systematic review of the literature. 02-Sep-2022;13:395. Available from: https://surgicalneurologyint.com/surgicalint-articles/11847/
Abstract
Background: Liponeurocytomas are rare neurocytic neoplasms that most often arise in the posterior fossa and affect individuals in the third and fifth decades of life. Most reported cases of this unique tumor in the literature have described a favorable clinical prognosis without recurrence. However, increasing reports of recurrent cases prompted the World Health Organization, in 2016, to recategorize the tumor from Grade I to the less favorable Grade II classification. We conducted a systematic review to identify recurrent cases of this unique tumor and to summarize differences between the primary and recurrent cases of liponeurocytoma.
Methods: A systematic review exploring recurrent liponeurocytoma cases was conducted by searching the PubMed, Google Scholar, and Scopus databases for articles in English. Abstracts from articles were read and selected for full-text review according to a priori criteria. Relevant full-text articles were analyzed for symptoms, imaging, location, histological, pathological, treatment, and recurrence-free time between the primary and recurrent cases.
Results: Of 4392 articles, 15 articles accounting for 18 patients were included (level of evidence: IV) in the study. Recurrence-free time decreased from an average of 82 months between the primary tumor resection to first recurrence to 31.3 months between the first and second recurrence. Recurrent tumors demonstrated increased pleomorphic neural cells, necrosis, vascular proliferation, and MIB-1 index when compared to the primary tumor. Several cases also demonstrated decreased lipidizing components when compared to the primary tumor, further indicating increased dedifferentiation. The primary treatment for this tumor was surgical resection with occasional adjunctive radiotherapy.
Conclusion: Recurrent cases of liponeurocytoma have features of increased malignant proliferation compared to the primary cases. The standard treatment for these primary and recurrent tumors is gross total resection. The role of adjunctive radiotherapy remains a matter of debate.
Keywords: Liponeurocytoma, Neurocytic neoplasm, Recurrent, Systematic review
INTRODUCTION
Liponeurocytomas are rare and slow-growing tumors of neuroectodermal origin first described by Bechtel et al. in 1978 who, on histological examination, reported mixed mesenchymal and neuroectodermal composition.[
In response to increasing reports of this unique pathology, in 2000, the World Health Organization (WHO) described liponeurocytoma as a separate mixed neuronal and glial tumor and categorized it as WHO Grade I, reflecting its favorable prognosis. However, as reports of recurrence after resection mounted, in 2016, the tumor was recategorized as the WHO Grade II.[
Over 80 cases of liponeurocytomas have been reported in the literature. Of those, 18 cases demonstrate recurrence following resection of the primary tumor.[
Here, we report a case of liponeurocytoma which recurred following two gross total resections (GTRs), along with a review of the 18 previously reported cases of recurrent liponeurocytoma with the hope of improving understanding of histopathological changes that occur with recurrence as well as guiding treatment and surgical decision-making in the treatment of this rare but intransigent entity.
MATERIALS AND METHODS
Case description
A 55-year-old man with a history of a left cerebellar liponeurocytoma resected at an outside facility 9 years prior was subsequently lost to follow-up. He presented to our institution’s emergency center with 2 months of severe bifrontal and occipital headaches as well as loss of balance with a predilection to falling toward his left. The patient’s physical examination was notable for mild dysmetria on finger-to-nose test (L>R) as well as mild ataxia with heel to shin (L>R).
Magnetic resonance imaging (MRI) revealed a mixed solid and cystic mass in the left cerebellum measuring 4.4 × 2.6 × 3.2 cm with surrounding vasogenic edema and associated mass effect with partial effacement of the fourth ventricle. The mass was T1 iso-to-hypointense and heterogeneously hyperintense on T2 [
Figure 1:
Magnetic resonance imaging demonstrates (a-d) presumptive recurrent liponeurocytoma (white arrow) as a heterogeneous solid and cystic mass in the left cerebellum abutting the left sigmoid sinus. (a-c) On axial, coronal, and sagittal T1-weighted imaging the lesion was isointense to the cortex with areas of hypointensity (not shown) with heterogeneous enhancement. The tumor was well marginated with minimal edema and without obstructive hydrocephalus. (d) Axial T2-weighted imaging shows heterogeneous hyperintensity of the tumor. Postoperative T1 axial, coronal, and sagittal (e-g); and T2 axial (h) images show complete resection of the mass.
Pathologic analysis revealed monotonous neurocyte-like cell proliferation, vague rosetting, and focal vascular proliferation. Multifocal fat droplets with adipocyte formation were also seen. No necrosis or mitotic activity was identified [
Three years postoperatively, routine surveillance MRI revealed a recurrent tumor measuring 2.1 × 2.0 × 2.9 cm in the left cerebellar hemisphere accompanied with mass effect on the left inferior sigmoid sinus, with associated loss of flow void in the distal left transverse sinus, proximal sigmoid sinus, and left jugular bulb. The mass was hyperintense on T1 and isointense on T2 [
Figure 3:
Magnetic resonance imaging (MRI) demonstrates (a-d) recurrence of the liponeurocytoma (white arrow) occupying the left cerebellum. (a-c) On axial, coronal, and sagittal T1-weighted imaging, the lesion was hyperintense compared with the cortex. The tumor was well marginated without edema or obstructive hydrocephalus. (d) Axial T2-weighted imaging of the tumor is isointense to the cortex (not shown), with increased heterogeneous enhancement as compared to the initial recurrence. Postoperative T1 axial, coronal, and sagittal (e-g); and T2 axial (h) MRI images demonstrate no radiographic evidence of residual disease.
Given that the patient was symptomatic, the patient was once again offered surgical resection. He underwent a redo left retrosigmoid craniotomy for resection of his tumor. At surgery, we found a largely well-encapsulated tumor. The tumor was initially internally debulked and then removed in piecemeal fashion. The patient had an unremarkable postoperative period. Postoperative MRI showed no evidence of residual tumor.
Pathologic analysis of the recurrent tumor demonstrated reduced lipomatous components compared to the prior pathology. Moreover, focal necrosis of the tumor, increased vascular proliferation, and increased cellularity were all noted on these tissue specimens, and the MIB-1 proliferation index had increased to 7.4%. Immunohistochemical marker phenotype remained unchanged [
Figure 4:
Histopathologic slides of the second recurrent liponeurocytoma (a) show small, microscopic collections of fat with increased vascularity and cellularity compared to the first recurrent tumor. (b) Small tumor cells with mixed lipomatous differentiated neoplastic cells express synaptophysin. (c) Axons of the surrounding central nervous system tissue express neurofilament. (d) The Ki-67 proliferation index (as determined by MIB-1 staining) is 7.4%.
Literature search
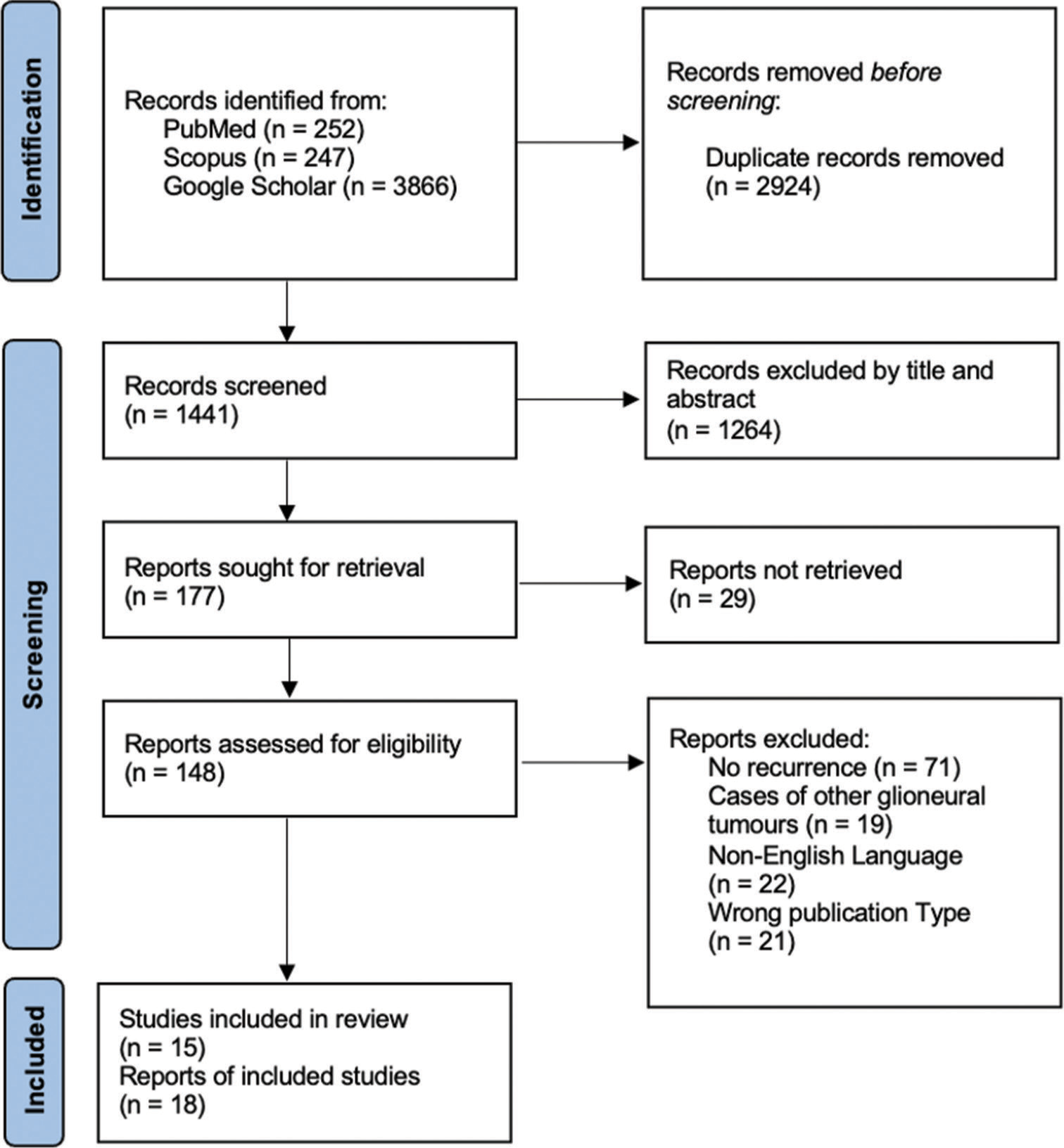
We performed a systematic review of recurrent liponeurocytoma following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines [
Duplicated records were excluded once the search was completed. Studies were subsequently screened based on title and abstract. The remaining articles were screened by a full-text review for final inclusion. Studies were selected for final review based on the following inclusion criteria: case report or peer-reviewed original research detailing a cohort of patients who underwent treatment for liponeurocytoma more than once (demonstrating recurrence), English language, and full-text availability. Studies other than full-length articles such as abstracts, posters, and editorials were excluded from the study. Two reviewers (D.S. and P.P) independently screened all articles and disagreements were reconciled by discussion. Data extraction was performed by two reviewers (D.S. and C.E.) based on prespecified criteria. Data of interest included identifying demographic characteristics and symptoms of individuals with recurrent liponeurocytoma; findings on imaging, pathology, and immunohistochemistry in the primary and recurrent tumors; treatment of primary and recurrent tumors; and time between the primary tumor and recurrence and between recurrences.
RESULTS
Our literature search identified 18 previously reported cases of recurrent liponeurocytoma [
The time between the primary tumor removal and detection of recurrence ranged from 8 months to 15 years with an average time for recurrence of 82 months. GTR of the recurrent tumor was performed in 13 cases (72.2%) and STR was performed in 5 cases (27.8%). Radiotherapy was employed in three cases for the treatment of the recurrent tumor. Seven of the previously reported patients, like our patient, experienced a second recurrence of their liponeurocytoma. Four of these patients had undergone complete resection of the first recurrence; three had undergone STR of the first recurrence. A second recurrence was not seen in patients who had been treated with radiotherapy.
The time between the first and second recurrence ranged from 3 months to 5 years with an average time for recurrence of 31.3 months. Five patients (71.4%) with a second recurrence were treated with a GTR of the tumor, 1 patient (14.3%) was treated with a STR supplemented with a standard medulloblastoma chemotherapy regimen, and 1 (14.3%) patient was treated with Gamma-Knife surgery. No more than 2 recurrences of the tumor have been reported in the literature.
Histological examination of the tumor was, for the most part, consistent between the primary tumor and the recurrent tumors. In six recurrent tumors (three from the first recurrence and three from the second recurrence), histological examination showed increased pleomorphic neural cells and decreased lipidized areas when compared to the primary tumor. In three recurrent tumors (one from the first recurrence and two from the second recurrence), histological examination showed increased lipidized areas when compared to the primary tumor. No necrosis or vascular proliferation was seen in the primary tumor. Necrosis and vascular proliferation were increased in six recurrent tumors (five from the first recurrence and one from the second recurrence). Increased mitosis from the primary tumor was described in five recurrent tumors (three from the first recurrence and two from the second recurrence). The Ki-67/MIB-1 proliferation index was described in 12 primary tumor cases with a mean of 5.08%. The Ki-67/ MIB-1 proliferation index was reported after the first tumor recurrence in 10 cases with a mean of 9.08%. The Ki-67/MIB-1 proliferation index was reported after the second tumor recurrence in four cases with a mean of 8.25%.
DISCUSSION
Liponeurocytomas are rare neurocytic neoplasms that most frequently arise in the posterior fossa and affect individuals in the third and fifth decades of life, and mostly demonstrate favorable clinical prognosis.[
Radiologically, liponeurocytoma share features seen in other cystic and neurocytic neoplasms of the central nervous system. They are usually described, like our first recurrence, as iso-to-hypointense on T1-weighting imaging and hyperintense on T2-weighted imaging.[
Histologically, liponeurocytomas are characterized by small and poorly differentiated neurocytic cells with focal areas of intermixed neuroepithelial cells with lipid accumulation. The tumor cells are usually described as round to oval.[
Recurrence in liponeurocytoma is rare. In a systematic review of 73 patients with liponeurocytoma, Gembruch et al. found that 14 or 28.6% had a primary tumor recurrence. Moreover, they found that six or 8.2% of patients experienced a second tumor recurrence.[
Complete surgical resection has been described as the preferred treatment for patients with liponeurocytoma. Despite this, Gembruch et al. found that of the 49 patients in their cohort that received complete surgical resection of the tumor, six experienced tumor recurrence. Radiotherapy has been discussed as a viable adjunctive therapy in patients with liponeurocytoma. Gembruch et al. found no recurrence in eight patients who received adjunctive radiotherapy paired with complete resection and only found one case of recurrence in six patients who received adjunctive radiotherapy paired with incomplete resection.[
Limitations
There are several limitations to this study. Although our research relied on three main databases, PubMed, Google Scholar, and Scopus, yielding over 4365 articles, there is a chance that the literature search is not entirely complete. This is especially true for this topic as liponeurocytoma was not established as a separate glioneuronal tumor until 2000. Thus, while our search accounted for other names used to describe this pathology, it is still likely that the results were not comprehensive. Moreover, only studies in English were included in the study, potentially excluding other regions where this pathology may be more common. In addition, because of the nature of this review, any errors in diagnosis, radiological examination, or pathological examination related to the included cases would bias our conclusions. Furthermore, the quality of evidence was moderate to low, as all the studies used in the review were case reports with a level of evidence of V and no randomized trials or cohort and case–control studies were identified. Another limitation of this study is the heterogeneity of the data collected in the case reports and case series. For instance, many cases did not have specific information on tumor imaging, size, pathology, or immunohistochemistry in the primary and recurrent tumors.
CONCLUSION
The present case is the 19th reported case of recurrent liponeurocytoma and only the eighth case with more than 1 recurrence following treatment. This paper highlights the noted radiological and histological differences between the primary and recurrent tumors and points to a trend of increased malignant proliferation between primary and recurrent tumors. The standard treatment for these tumors is complete resection. The role of adjunctive radiotherapy remains an area of investigation, but the data thus far indicate that at least in the case of histologically aggressive appearing tumors, adjunctive radiation treatment is likely warranted.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alkadhi H, Keller M, Brandner S, Yonekawa Y, Kollias SS. Neuroimaging of cerebellar liponeurocytoma: Case report. J Neurosurg. 2001. 95: 324-31
2. Anghileri E, Eoli M, Paterra R, Ferroli P, Pollo B, Cuccarini V. FABP4 is a candidate marker of cerebellar liponeurocytomas. J Neurooncol. 2012. 108: 513-9
3. Bechtel JT, Patton JM, Takei Y. Mixed mesenchymal and neuroectodermal tumor of the cerebellum. Acta Neuropathol. 1978. 41: 261-3
4. Belka C, Budach W, Kortmann RD, Bamberg M. Radiation induced CNS toxicity--molecular and cellular mechanisms. Br J Cancer. 2001. 85: 1233-9
5. Bonneville F, Cattin F, Marsot-Dupuch K, Dormont D, Bonneville JF, Chiras J. T1 signal hyperintensity in the sellar region: Spectrum of findings. Radiographics. 2006. 26: 93-113
6. Buccoliero AM, Caldarella A, Bacci S, Gallina P, Taddei A, Di Lorenzo N. Cerebellar liponeurocytoma: Morphological, immunohistochemical, and ultrastructural study of a relapsed case. Neuropathology. 2005. 25: 77-83
7. Chakraborti S, Mahadevan A, Govindan A, Yasha TC, Santosh V, Kovoor JM. Supratentorial and cerebellar liponeurocytomas: Report of four cases with review of literature. J Neurooncol. 2011. 103: 121-7
8. Cohen-Inbar O, Vlodavsky E, Zaaroor M. The natural history and treatment guidelines of cerebellar liponeurocytoma-a case report. Open J Mod Neurosurg. 2011. 1: 10-6
9. Gembruch O, Junker A, Mönninghoff C, Ahmadipour Y, Oppong MD, Sure U. Liponeurocytoma: Systematic review of a rare entity. World Neurosurg. 2018. 120: 214-33
10. George DH, Scheithauer BW. Central liponeurocytoma. Am J Surg Pathol. 2001. 25: 1551-5
11. Giangaspero F, Cenacchi G, Roncaroli F, Rigobello L, Manetto V, Gambacorta M. Medullocytoma (Lipidized medulloblastoma): A cerebellar neoplasm of adults with favorable prognosis. Am J Surg Pathol. 1996. 20: 656-64
12. Guan JT, Geng YQ, Cheng Y, Guo YL, Wu RH. Magnetic resonance imaging of cerebellar liponeurocytoma: A case report and review of the literature. Neuroradiol J. 2012. 25: 331-6
13. Hermann B, Woznica M, Kloc W, Borkowski P, Libionka W, Izycka-Swieszewska E. Cerebellar liponeurocytoma with atypical histological features-a rare example of a glioneuronal tumor. Folia Neuropathol. 2017. 55: 227-34
14. Jenkinson MD, Bosma JJ, Du Plessis D, Ohgaki H, Kleihues P, Warnke P. Cerebellar liponeurocytoma with an unusually aggressive clinical course: Case report. Neurosurgery. 2003. 53: 1425-7
15. Jouvet A, Lellouch-Tubiana A, Boddaert N, Zerah M, Champier J, Fèvre-Montange M. Fourth ventricle neurocytoma with lipomatous and ependymal differentiation. Acta Neuropathol (Berl). 2005. 109: 346-51
16. Khatri D, Bhaisora KS, Das KK, Behari S, Pal L. Cerebellar liponeurocytoma: The dilemma of multifocality. World Neurosurg. 2018. 120: 131-7
17. Konovalov AN, Konovalov NA, Pronin IN, Shishkina LV, Zolotova LI, Yakovlenko YG. Multiple primary liponeurocytoma of the central nervous system. Zh Vopr Neirokhir Im N N Burdenko. 2015. 79: 87-96
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009. 339: b2700
19. Limaiem F, Bellil S, Chelly I, Bellil K, Mekni A, Jemel H. Recurrent cerebellar liponeurocytoma with supratentorial extension. Can J Neurol Sci. 2009. 36: 662-5
20. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
21. Pelz D, Khezri N, Mainprize T, Phan N, Keith J, Bilbao J. Multifocal cerebellar liponeurocytoma. Can J Neurol Sci. 2013. 40: 870-2
22. Radke J, Gehlhaar C, Lenze D, Capper D, Bock A, Heppner FL. The evolution of the anaplastic cerebellar liponeurocytoma: Case report and review of the literature. Clin Neuropathol. 2015. 34: 19-25
23. Romano N, Federici M, Castaldi A. Imaging of extraventricular neurocytoma: A systematic literature review. Radiol Med. 2020. 125: 961-70
24. Shen S, Tang Y, Yang R, Zhou D. Typical radiological features of a rare lateral ventricular liponeurocytoma. Ann Neurol. 2021. 90: 851-2
25. Soylemezoglu F, Soffer D, Onol B, Schwechheimer K, Kleihues P. Lipomatous medulloblastoma in adults. A distinct clinicopathological entity. Am J Surg Pathol. 1996. 20: 413-8
26. Wolf A, Alghefari H, Krivosheya D, Staudt MD, Bowden G, Macdonald DR. Cerebellar liponeurocytoma: A rare intracranial tumor with possible familial predisposition. Case report. J Neurosurg. 2016. 125: 57-61
27. Xu L, Du J, Wang J, Fang J, Liu Z, He Y. The clinicopathological features of liponeurocytoma. Brain Tumor Pathol. 2017. 34: 28-35