Radioterapia más temozolomida o PCV en pacientes con oligodendroglioma anaplásico con codeleción 1p19q
Introducción. La radioterapia con procarbacina, lomustina y vincristina (PCV) mejora la supervivencia global en pacientes con oligodendroglioma anaplásico con codeleción 1p19q, pero no está disponible en América Latina.
Pacientes y métodos. Análisis retrospectivo comparando dos protocolos diferentes, radioterapia más temozolomida o PCV, en pacientes con oligodendroglioma anaplásico con codeleción 1p19q. Los objetivos primarios fueron la supervivencia global y la supervivencia libre de progresión, y el objetivo secundario, la respuesta radiológica.
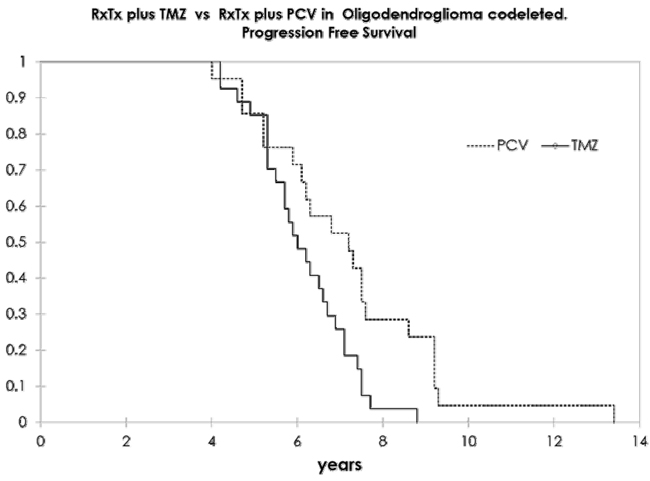
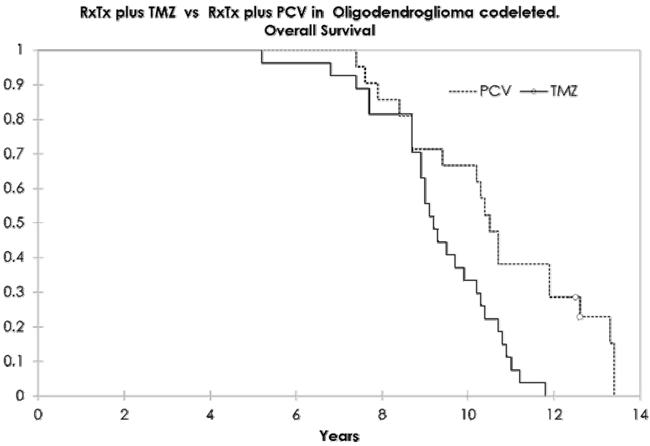
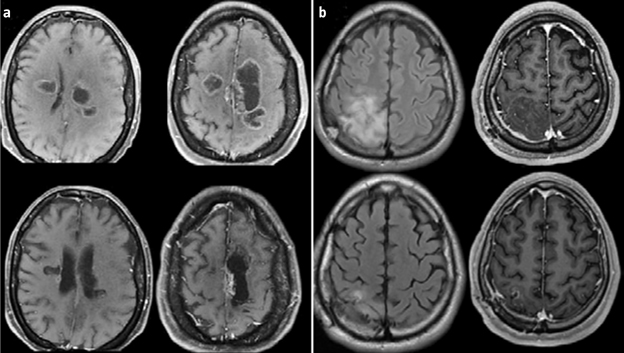
Resultados. Se incluyó a 48 pacientes, 26 de ellos varones (54,1%), con una edad media de 43 años (rango: 19-66 años). Veintiún pacientes recibieron PCV, y 27, temozolomida. Las características iniciales no tuvieron diferencias entre los grupos. La supervivencia libre de progresión y la supervivencia global en el grupo con PCV fueron de 7,2 y 10,6 años, y en el grupo de temozolomida, de 6,1 y 9,2 años, respectivamente, unos resultados estadísticamente significativos. Hubo respuesta radiológica en el 80,9% en el brazo de PCV y el 70,2% en el brazo de temozolomida. El análisis multivariado de Cox mostró como único parámetro significativo el uso del protocolo PCV. El grado de toxicidad 3-4 estuvo presente en el 42,8% en el brazo de PCV y en el 11,1% en el brazo de temozolomida.
Conclusiones. La estrategia más común en América Latina es la sustitución de PCV por temozolomida. Este estudio retrospectivo mostró una eficacia superior de PCV que de la temozolomida. La diferencia obliga a la comunidad latinoamericana a hacer un esfuerzo colectivo para poder tener acceso a los medicamentos para su uso como primera línea de tratamiento.
Palabras clave. Codeleción 1p19q. Oligodendroglioma. PCV. Temozolomida.
|
 Castellano
Castellano
 English
English