Abstract
Purpose
To analyze the clinical characteristics of abdominal pregnancy, and to explore the diagnosis and prognosis of different treatment methods.
Methods
The cases of patients with abdominal pregnancy admitted to Peking Union Medical College Hospital between January 1, 1989 and January 1, 2021, were analyzed retrospectively.
Results
The median age of 17 patients was 34 years (22–42 years); the median gestational duration was 57 days (from 41 days to 32 weeks). Among all 17 patients, 15 (88.24%) presented with abdominal pain. The implantation sites of the gestational sac included the bladder peritoneal reflection, anterior wall of the rectum, omentum, serous membrane of the uterus, and inside or on the surface of uterosacral ligament. In all, only 29.41% cases (5/17) were diagnosed before surgery. All 17 patients were treated via surgery. Further, 58.82% (10/17) patients recovered without complications, 29.41% (5/17) developed fever, 5.88% (1/17) underwent reoperation because of intra-abdominal bleeding, and 5.88% (1/17) developed double lower limb venous thrombosis. All 17 patients survived.
Conclusion
The preoperative diagnosis rate of abdominal pregnancy is low. Planting sites in the pelvic peritoneum and pelvic organs are more common than the others. Laparoscopic surgery in the first trimester of pregnancy can achieve better therapeutic effects. However, the blood supply of the placenta should be fully evaluated before surgery. When it is expected that attempts to remove the placenta will cause fatal bleeding, the placenta can be left in place, but long-term close follow-up should be paid attention to.
Similar content being viewed by others
Introduction
As a pathological pregnancy, ectopic pregnancies accounts for approximately 1–2% of all pregnancies [1]. Among them, in more than 90%, the implantation site is in the fallopian tube. In abdominal pregnancies, the gestational sac is implanted in the peritoneal cavity outside the uterine cavity or fallopian tube; these cases account for approximately 1.4% of all ectopic pregnancies [2]. The implantation sites in abdominal pregnancy in previous reports have included the following: the omentum, peritoneum of pelvic and abdominal cavity, uterine surface and abdominal organs such as spleen, intestine, liver, large blood vessels in the abdominal cavity, diaphragm, and others [3]. The symptoms and signs in patients vary according to the implantation site. If the implantation site is in the pelvic cavity, early diagnosis is easily confused with tubal ectopic pregnancy [4], and only 20–40% of cases are diagnosed before surgery [5]. Advanced abdominal pregnancy (AAP), that is, an abdominal pregnancy after 20 weeks of gestation, caused by the implantation of an abnormal placenta, can cause severe maternal postpartum hemorrhage and coagulopathy, which could lead to death in severe cases [6]. Accordingly, maternal mortality rate is approximately seven times higher in abdominal pregnancies compared with that in other ectopic pregnancies [7].
In this article, we report a case of abdominal pregnancy at 3 months. The pregnancy was thought to have been terminated at a local hospital during the first trimester, but an abdominal pregnancy was soon after discovered in the second trimester. We share the medical history, imaging findings, diagnosis, and treatment of the patient, and reviewed and summarized the characteristics, diagnosis, and treatment of 17 cases, including the current case, of abdominal pregnancy at our hospital's obstetric center in the past 35 years, hoping to provide a new basis for abdominal pregnancy management.
Case report
A 39-year-old woman, G7P3, with 3 healthy children. Her last delivery was in 2013. Approximately 45 days ago, an ultrasound at a local clinic revealed a 2-month intrauterine pregnancy, and a medical abortion was conducted subsequently on patient’s request. This was followed with uterine curettage because of abortion failure. The medical records of the patient could not be traced back. After the operation, the patient had vaginal spotting for 5 days, and no more vaginal bleeding or menstrual cramps at the time of consultation. One month after the operation, the patient presented with palpitation and nausea. The local hospital’s ultrasound displayed that the uterus was 8.4 × 6.4 × 6.1 cm large, and the gestational sac was 11.2 × 10.5 × 8.1 cm, and was cited on the left posterior uterus. The fetus was visible inside; the double parietal diameter was 3.2 cm, and the amniotic fluid depth was 3.3 cm, indicating an abdominal pregnancy. Therefore, the patient was transferred to our hospital.
The patient’s body temperature was 37.5 °C when admitted to our hospital, and she had intermittent mid-abdominal pain. No lower abdominal pain or swelling or vaginal bleeding was observed. Laboratory examinations revealed that the white blood cells were in the normal range, but the proportion of neutrophils had increased to 84.6%, accompanied by an increase in hsCRP of 31.83 mg/L, as well as mild hypokalemia, hypoproteinemia, and mild anemia. Further, β-HCG level was 55,264.9 IU/L. An ultrasound revealed that the uterus was enlarged, the endometrial thickness was 0.8 cm, and the gestational sac was located on the left rear side of the uterus. A formed fetus was seen inside, with a fetal BPD of 3.6 cm, fetal abdominal circumference of 10.6 cm, and fetal femur length of 2.2 cm. The fetal heartbeat was visible, and amniotic fluid depth was approximately 4.0 cm. The placenta was located in the right front of the left iliac blood vessel, with a thickness of approximately 3.5 cm and a length of approximately 16.1 cm. There was no obvious free liquid in the pelvis. The diagnosis was confirmed after magnetic resonance imaging (MRI) (Fig. 1). On MRI, the posterior, right, and anterior pelvic short T1 signals around the placenta were considered as bloody effusions. Vascular ultrasound and angiography confirmed that the blood supply to the placenta came from the left uterine artery and left ovarian artery. Gelatinous sponge particles were used to embolize the left uterine artery and left ovarian artery, and the embolization was smoothly conducted. After 1 h, ultrasound imaging indicated that the fetal heart had stopped beating. Except for slight abdominal pain, there were no other discomforts to the patient later that night.
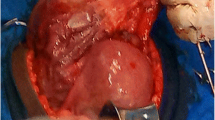
After sufficient preparation, the patient was sent to the operating room for an exploratory laparotomy. During the operation, the gestational sac was seen behind the uterus, with a formed fetus in it, and the amniotic fluid was clear (Fig. 2). The fetus was taken out after ligating the umbilical cord, and the appearance of the fetus had no deformity. After observing for 30 min, except for slight blood oozing from the edge of the placenta, no other evidence of placental abruption was observed. We decided to leave the placenta at site and close the abdomen. The amount of bleeding during surgery was estimated to be 50 ml, and no blood transfusion was required. After the operation, the patient’s body temperature increased to 38.1 ℃, but soon decreased after antibiotic treatment. There was no decrease in hemoglobin levels on the 1st, 3rd, and 8th day after the operation. On the 10th day after the operation, ultrasound-revealed medium echo at the back of the uterus was approximately 7.8 cm long and 4.5 cm thick. There were scattered small echoes but no obvious blood flow signals within. The patient was discharged from the hospital and followed up regularly at the outpatient clinic. The blood β-HCG returned to normal on the 26th day after the operation, but ultrasound still showed a moderate echo of 6.2 × 5.2 × 4.3 cm behind the uterus. The patient has no discomfort and is still under close follow-up.
Case series
Methods
We reviewed the cases of abdominal pregnancy that were treated at our hospital and confirmed by intraoperative findings and pathological results. That is, all the cases with “abdominal pregnancy” in the discharge diagnosis were searched in the medical record retrieval system, and the cases with unclear diagnosis and repeated hospitalization were excluded. A total of 17 cases including the current case have been treated at our hospital, and the pregnancy history, diagnosis, treatment, and complications of these patients were analyzed, with an aim to achieve a reference for this case and other similar cases.
Results
Basic information
All 17 cases occurred between 1989 and 2021, in this period, the total number of deliveries and ectopic pregnancies diagnosed in our unit were 62,121 and 9095, respectively. Abdominal pregnancies accounts for approximately 0.19% of all ectopic pregnancies. Among all 17 abdominal pregnancy patients, the median age was 34 years (22–42 years), and the median gestation was 57 days (41 days to 32 weeks). All patients had symptoms, and 15 of 17 (88.24%) had abdominal pain; 7 of 17 (41.18%) patients had vaginal bleeding. The implantation sites of the gestational sac were bladder peritoneal reflection, anterior rectal wall, greater omentum, uterine serosa, medial uterosacral ligament, or uterosacral ligament (Fig. 3). Further, 29.41% (5/17) of patients were diagnosed before surgery, and the other 70.59% (12/17) were misdiagnosed with tubal pregnancy or unexplained intra-abdominal hemorrhage (patients in early pregnancy) and central placenta previa (patients in third trimester) before surgery. The primary method of preoperative evaluation was ultrasound, and it was not until 2020 that MRI and angiography were first added to preoperative diagnostic procedures for suspected abdominal pregnancies (Fig. 4). All 17 patients were treated by laparoscopy or open surgery, no conservative/non-surgical management. Subsequently, 58.82% (10/17) recovered without complications after treatment, 29.41% (5/17) developed fever, which improved after antibiotic treatment, 5.88% (1/17) developed intra-abdominal hemorrhage and hemorrhagic shock, and had to undergo a reoperation, and 5.88% (1/17) had venous thrombosis in both lower extremities and needed additional anticoagulation therapy. No deaths occurred (Table 1).
Non-advanced abdominal pregnancy
Diagnosis and treatment
The gestational age of 13 of 17 (76.47%) patients was less than 20 weeks; of these 2 patients were diagnosed before treatment, and both were in the second trimester and the fetal heartbeat could be seen on imaging. Both these patients underwent laparotomy. Among these two, one patient developed venous thrombosis in both lower extremities after operation. Among the remaining 11 cases that were not diagnosed before operation, three were presumed to be secondary to a recent tubal ectopic pregnancy, although the abnormal tube was removed in two cases during a previous operation; the remaining 8 were primary cases. In addition, all 11 patients in the first trimester were not diagnosed by imaging examinations before surgery; 10 of them had abdominal pain, but 7 of these 10 (70%) were misdiagnosed with tubal pregnancy; four patients received blood transfusion because of gestational sac implanting site rupture and suffered heavy bleeding. The pregnancy tissue was successfully removed in all 11 patients through laparoscopic surgery, and blood β-HCG levels fell to the normal range within 1 month after the operation (Table 2).
Risk factors
In all, 9 of 13 (69.23%) patients with a gestational age of less than 20 weeks had high risk factors for an abdominal pregnancy: 1 patient was fertilized by ovulation induction because of primary infertility; 2 had intrapelvic adhesions; 2 had cysts in the fallopian tubes; 2 had a history of pelvic surgery, particularly fallopian tube surgery; 1 had a history of uterine cavity operation in early gestation and 3 had a history of tubal ectopic pregnancy confirmed by laparoscopy and pathology.
Advanced abdominal pregnancy
Diagnosis and treatment
Overall, 23.53% (4/17) of patients had AAP, that is, gestational age > 20 weeks. All four cases were observed in 2000s and before. All four patients had symptoms of abdominal pain, three were diagnosed by imaging examinations before surgery, and all received blood transfusions because of excessive bleeding during the perioperative period. Among the four patients, there was one fetal malformation, one stillbirth, and one live birth. With regard to the placenta, in two cases, the placenta was attached to a fallopian tube and ruptured uterus with remnant uterine horn, respectively. This is speculated to be secondary to tubal pregnancy and remnant uterine horn pregnancy. The four patients were treated via laparotomy. In one patient, the placenta attached to the left fallopian tube was removed during the operation, and the placenta was left in site in three other cases. However, one patient showed intra-abdominal hemorrhage and hemorrhagic shock on the same day; thus, the patient needed another surgical treatment. During the second operation, it was found that the placenta remained in situ attached to a ruptured residual uterine horn, which was not accurately recognized by imaging before and during the first operation. Another patient underwent another laparotomy 26 months later because of another ectopic pregnancy ruptured in the left fallopian tube, and the remaining placenta was removed during this operation. All four patients developed fever after operation, but antibiotic treatment was effective, and they were discharged after a good recovery.
Risk factors
Overall, 50% (2/4) of patients had high risk factors for abdominal pregnancy in this patient group. Among the two patients, one had a history of uterine cavity operation in early pregnancy and the other was naturally conceived after four years of infertility.
Discussion
In the past 22 years, the ratio of ectopic pregnancy to delivery numbers in our hospital was about 14.64% (9095/62121), which is higher than that reported in other literatures. This may be because our hospital is Chinese National Clinical Research Center for Obstetrics & Gynecologic Diseases, and accepts referral patients with difficult and severe diseases in obstetrics and gynecology from Chinese capital and surrounding areas, including rare ectopic pregnancies such as cesarean scar pregnancy, cervical pregnancy, etc. In addition, some patients suspected of ectopic pregnancy in other hospitals hope to receive further diagnosis and treatment in our hospital and voluntarily request referral to our hospital. Therefore, these data are not representative of the level of ectopic pregnancy in the region over the 22-year period.
An abdominal pregnancy can be primary (wherein the blastocyst is directly implanted on the surface of the peritoneum or the viscera of the abdominal cavity) or secondary (wherein the embryo falls from the fallopian tube into the abdominal cavity). In our study, there are three cases of tubal ectopic pregnancy that occurred not long ago. Two cases of AAP in this study, where the placenta was attached to a fallopian tube/ruptured residual uterine horn, could be considered secondary or uterine cavity manipulation in the first trimester (CASE 2 and CASE 16), which may cause iatrogenic perforation of the uterus and free the gestational sac into the abdominal cavity [8]; however, this cannot be confirmed. The diagnostic criteria for primary abdominal pregnancy have been proposed by Studdiford [9] in 1942: (1) both fallopian tubes and ovaries are normal; (2) no uterine-peritoneal fistula formation; (3) Pregnancy only exists in the abdominal cavity; and (4) there is no possibility of tubal pregnancy. Ten of our cases can be considered primary according to this standard. Although we consider the diagnostic criteria to be ambiguous in the definition of “no possibility of tubal pregnancy”, since in our case, most abdominal pregnancies were implanted in the pelvic cavity and both fallopian tubes and ovaries were normal. But whether they had a tubal pregnancy followed by a complete miscarriage into the pelvic cavity was not identifiable. Our recommendation is that when β-HCG is elevated and the gestational sac cannot be located intrauterine, ultrasound or MRI should be attempted first in the pelvic cavity, including the fallopian tube and surrounding area, regardless of whether there had been a tubal pregnancy.
The risk factors of abdominal pregnancy include fallopian tube injury, pelvic inflammatory disease, endometriosis, and pluripara among others [10]. In a literature review of the case reports of abdominal pregnancy after in vitro fertilization-embryo transfer [11], 37% of cases of abdominal pregnancy have a history of tubal ectopic pregnancy, and 61% of cases have anatomical/structural infertility, with fallopian tube factors being the most common; the incidence in fresh embryo transfer (71%) is much higher than that in frozen embryo transfer (11%). In addition, there are also reports that the use of cocaine may be a risk factor for abdominal pregnancy [12]; further, the incidence in non-industrialized countries is higher than that in industrialized countries [13].
The clinical symptoms of an abdominal pregnancy are uncertain. According to our research, most patients have abdominal pain and vaginal bleeding [14]. In AAP, abdominal pain may manifest as fetal movement pain [15]; other symptoms include placenta and fetal position abnormalities, abnormal cervix position, and failure to induce labor [16]; some patients may be admitted for shock cause by rupture of ectopic pregnancy lesions. Excessively elevated alpha-fetoprotein levels in laboratory tests can also be used to guide diagnosis [17]. Imaging examinations such as ultrasound, MRI, and computed tomography (CT) are essential in confirming the diagnosis, and can be used to evaluate the position of the gestational sac, blood supplies, implantation site, and bleeding lesions [18]; even in our treatment experience, vascular ultrasound and angiography could also be used to evaluate the blood supply of the placenta. Only 50% of early abdominal pregnancy can be diagnosed by ultrasound [19], but when combined with serum β-HCG levels, the sensitivity of ultrasound increases. MRI, as a multi-planar, multi-parameter imaging method with high resolution of soft tissues and no radiation, can accurately visualize the intra-abdominal structure, can show the anatomical relationship among the fetus, placenta, and the maternal organs in detail, and can also show vascular invasion [20]. Although the safety of MRI plain scan during pregnancy has long been affirmed [21], the intravenous gadolinium contrast agent used in enhanced MRI is listed in the US Food and Drug Administration (FDA) drug classification Class C pregnancy drugs, and studies have shown that intrauterine gadolinium exposure is related to stillbirths, neonatal deaths, and various skin abnormalities [22]; therefore, when there is a possible intrauterine pregnancy or AAP wherein the fetus is expected to be preserved, enhanced MRI scans should be performed with caution. Although after undergoing imaging evaluations mentioned above, abdominal pregnancy may be diagnosed and treated early in areas with well-developed medical conditions, in areas with insufficient medical resources and inadequate prenatal care, abdominal pregnancy may be diagnosed at greater gestational age or even full-term [23]. In our case, all AAPs occurred in 2000 and before, which is consistent with the rapid development of our country.
Of the 17 cases of abdominal pregnancy in our study, only five (29.41%) were diagnosed before surgery, and the accuracy of diagnosis increased with the increase in gestational age and the appearance of fetal heart rate. Further, seven (41.18%) of the total patients required blood transfusion during the perioperative period; this percentage among AAP cases was 75%. All our patients were treated surgically. There are no standard treatment methods acknowledged, and no predictive standards for successful medical management [24]. Previous literature reports on the therapeutic regimen include conservative treatments and surgical treatments. Conservative treatments include selective placental vascular embolization, ultrasound-guided drug injection in the gestational sac, or maternal systemic drug therapy [25, 26]. Conservative treatments may need a long follow-up period. A previous study reported that a 14 week gestation was terminated by ultrasound-guided induction, and the fetus and placenta remained in site. The follow-up visit revealed that the gestational sac degraded very slowly, and only a small amount amniotic fluid volume was reduced at 9 months after surgery [27].
Surgical treatment is the most common treatment for abdominal pregnancy. Although laparotomy has an irreplaceable advantage over laparoscopic surgery in terms of rapid and adequate hemostasis, there are still many reports on laparoscopic surgery for the treatment of abdominal pregnancy [28], especially in early stages of the pregnancy, laparoscopic removal the ectopic pregnancy tissues can be tried [29], which achieved an excellent therapeutic effect in our case. However, before the operation, the site of implantation of the gestational sac should be fully assessed via imaging as much as possible, and a multidisciplinary team should cooperate to prepare for hemostasis and salvage, and to choose selective embolization, if necessary.
Abdominal pregnancies in the second and third trimesters were all treated through laparotomy, but because of the fear of massive bleeding after placental dissection [30] and maternal perinatal death [31]; further, in 4 (66.67%) of the six cases with the second and third trimester abdominal pregnancy, it was chosen to keep the placenta at the site. However, it is recommended to fully evaluate the position and blood supply of the placenta, because when medical care was inadequate, we had insufficient preoperative evaluation. This led to massive intra-abdominal hemorrhage and patient went in a hemorrhagic shock a few hours after the first laparotomy; the second operation proved that the placenta was attached to the ruptured rudimentary uterus and was partially abrupted. In this case, the placenta was resectable during the first operation. In addition, in the latest case, we tried to determine the blood supply to the placenta through a vascular ultrasound and angiography before surgery, and then embolized the placental blood vessel [32], which significantly reduced perioperative bleeding and avoided the need of a blood transfusion. In the case where the placenta was left in site, we chose to stop administering drugs and waited for self-absorption to avoid the use powerful drugs to cause rapid necrosis of the placenta, which can cause severe intra-abdominal infection [33]. However, in previous literature reports, some physicians believed that post-operative residual placenta still needs drug treatment, and tried to apply small-dose methotrexate systemic therapy for some time after surgery [34], which can also avoid complications such as infection and bleeding. Further, this same physician, after collecting relevant literature from the database and relevant data on the case, proposed that when the gestational sac is planted in a vascular-rich area such as the iliac vascular area, even if the residual placenta has no blood flow, the nearby blood vessels could be torn because of activity, causing massive bleeding [35]. Accordingly, he proposed that when the patient is stable and the placental blood flow stops approximately 3 months after the termination of pregnancy, the placenta should be surgically removed again to avoid the risk of further bleeding and infection. However, in our cases, the placenta left in site was followed up for a maximum period of 26 months, and during this period, the condition of patients was stable.
Interestingly, there is a striking similarity with the intrauterine placenta accreta spectrum cases and abdominal pregnancy, especially in the placenta management of AAP. Intentional Retention of the placenta (IRP) [36] is intentionally leaving placenta in the uterus after delivering the baby, including subsequent removal/non-removal of the placenta. Similar to AAP, the placenta accreta spectrum may be associated with invasion and damage to other organs. In addition, fertility preservation needs to be considered, as some women may wish for subsequent pregnancies. Some physicians believe that IRP is a reasonable option to reduce the risk of catastrophic bleeding because it avoids abundant blood supply of periuterine during the postpartum period [37], but other reports believe that it increases the rate of arterial embolism, infection, and the risk of re-hospitalization [38]. The difference in the treatment of AAP in placenta is that the risk of infection after placenta retention is lower than that of IRP due to the lack of direct communication with the outside world. However, with the lack of direct communication with the outside world, the placenta remains in the abdominal cavity, fatal hemorrhage is initially undetectable, and the patient may not be noticed until hemorrhagic shock.
According to reports in the literature, an abdominal pregnancy has a higher incidence of fetal malformations and perinatal mortality [6]. Among our four patients with AAP, there was one case of fetal malformation and one stillbirth, and only one live birth. Previous studies have analyzed literature reports of 39 cases of abdominal pregnancy, of which only two cases reported neonatal survival [39]. This may be related to the unstable blood supply to the placenta in the abdominal cavity and fetal stress deformity [40].
Abdominal pregnancy is a rare ectopic pregnancy. Although most abdominal pregnancies can be detected and terminated at an early stage with the popularization of prenatal care, its diagnosis and treatment are still a big challenge in areas with insufficient medical resources. In addition, for abdominal pregnancies in the second and third trimesters, especially AAP, conservative treatment methods and the treatment of forced in site placenta also need to be further supported and explored by evidence-based medical studies.
References
Pregnancy-Related Mortality Ratios, United States 1991–1999 CDC. MMWR vol 52, ss-2, February 21 2003
Atrash HK, Friede A, Hogue CJ (1987) Abdominal pregnancy in the United States: frequency and maternal mortality. Obstet Gynecol 69:333
Yang M, Cidan L, Zhang D (2017) Retroperitoneal ectopic pregnancy: a case report and review of the literature. BMC Pregnancy Childbirth. https://doi.org/10.1186/s12884-017-1542-y
Onan MA, Turp AB, Saltik A et al (2005) Primary omental pregnancy: case report. Hum Reprod 20:807
Masukume G (2013) Live births resulting from advanced abdominal extrauterine pregnancy, a review of cases reported from 2008 to 2013. Webmedcentral Com 5(1)
Sharma R, Puri M, Madan M et al (2012) Advanced live intra-abdominal pregnancy with good fetomaternal outcome: a case report. Int J Case Reports Images 3(11):5–7
Kassam M (2007) Abdominal pain in pregnancy. In: David KJ, Philips JS, Carl PW, Bernard G (eds) High risk pregnancy: management option. WB Saunders, London, pp 996–997
Fisch B, Peled Y, Kaplan B et al (1996) Abdominal pregnancy following in vitro fertilization in a patient with previous bilateral salpingectomy. Obstet Gynecol 88:642
Studdiford WE (1942) Primary peritoneal pregnancy. Am J Obstet Gynecol 44(3):487–491
Sato H, Mizuno Y, Matsuzaka S et al (2019) Abdominal pregnancy implanted on surface of pedunculated subserosal uterine leiomyoma: a case report. Case Reports Womens Health 24:e00149
Yoder N, Tal R, Martin JR (2016) Abdominal ectopic pregnancy after in vitro fertilization and single embryo transfer: a case report and systematic review. Reprod Biol Endocrinol: RBE 14:1–10
Audain L, Brown WE, Smith DM et al (1998) Cocaine use as a risk factor for abdominal pregnancy. J Natl Med Assoc 90(5):277–283
Nunyalulendho DN, Einterz EM (2008) Advanced abdominal pregnancy: case report and review of 163 cases reported since 1946. Rural Remote Health 8(4):1087
Varma R, Mascarenhas L, James D (2003) Successful outcome of advanced abdominal pregnancy with exclusive omental insertion. Ultrasound Obstet Gynecol 21(2):192–194
Paluku JL, Kalole BK, Furaha CM et al (2020) Late abdominal pregnancy in a post-conflict context: case of a mistaken acute abdomen—a case report. BMC Pregnancy Childbirth. https://doi.org/10.1186/s12884-020-02939-3
Tucker K, Bhardwaj NR, Clark E et al (2017) Delayed diagnosis and management of second trimester abdominal pregnancy. BMJ Case Rep 2017:bcr-2017-221433
Nassali MN et al (2016) A case report of an asymptomatic late term abdominal pregnancy with a live birth at 41 weeks of gestation. BMC Res Notes. https://doi.org/10.1186/s13104-016-1844-6
Si MJ, Gui S, Fan Q et al (2016) Role of MRI in the early diagnosis of tubal ectopic pregnancy. Eur Radiol 26(7):1971–1980
Chughtai F (2017) Twin abdominal pregnancy—a rare scenario. J Pakistan Med Assoc 67(5):793–795
Teng HC, Kumar G, Ramli NM (2007) A viable secondary intra-abdominal pregnancy resulting from rupture of uterine scar: role of MRI. Br J Radiol 80:134–136
Yip YP, Capriotti C, Talagala SL, Yip JW (1994) Effects of MR exposure at 1.5 T on early embryonic development of the chick. J Magn Reson Imaging 4(5):742–748
Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL (2016) Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 316(9):952–961
Aliyu LD, Ashimi AO (2013) A multicentre study of advanced abdominal pregnancy: a review of six cases in low resource settings. Eur J Obstet Gynecol Reprod Biol 170(1):33–38
Agha RA, Borrelli MR, Farwana R, Koshy K, Fowler A, Orgill DP, For theSCARE Group (2018) The SCARE 2018 statement: updating consensus Surgical CAseREport (SCARE) guidelines. Int J Surg 60:132–136
Moores KL, Keriakos RH, Anumba DO et al (2010) Management challenges of a live 12-week sub-hepatic intra-abdominal pregnancy. BJOG 117(3):365–368
Python JL et al (2016) Ultrasound-guided percutaneous management of splenic ectopic pregnancy—ScienceDirect. J Minim Invasive Gynecol 23(6):997–1002
Mitra AG, LeQuire MH (2003) Minimally invasive management of 14.5-week abdominal pregnancy without laparotomy: a novel approach using percutaneous sonographically guided feticide and systemic methotrexate. J Ultrasound Med 22:709
Okorie CO (2010) Retroperitoneal ectopic pregnancy: is there any place fornon-surgical treatment with methotrexate? J Obstet Gynaecol Res 36:1133–1136
Hajji A, Toumi D, Laakom O et al (2020) Early primary abdominal pregnancy: diagnosis and management. A case report. Int J Surg Case Rep 73:303–306
Cardosi RJ, Nackley AC, Londono J et al (2002) Embolization for advanced abdominal pregnancy with a retained placenta. A case report. J Reprod Med 47(10):861–863
Worley KC, Hnat MD, Cunningham FG (2008) Advanced extrauterine pregnancy: diagnostic and therapeutic challenges. Am J Obstet Gynecol 198(3):297.e1-297.e7
Cardosi RJ, Nackley AC, Londono J, Hoffman MS (2002) Embolization for advanced abdominal pregnancy with a retained placenta. A case report. J Reprod Med 47(10):861–863
Cetinkaya MB, Kokcu A, Alper T (2005) Follow up of the regression of the placenta left in situ in an advanced abdominal pregnancy using the Cavalieri method. J Obstet Gynaecol Res 31:22
Huang K, Song L, Wang L, Gao Z, Meng Y, Lu Y (2014) Advanced abdominal pregnancy: an increasingly challenging clinical concern for obstetricians. Int J Clin Exp Pathol 7(9):5461–5472
Masukume G, Sengurayi E, Muchara A, Much-eni E, Ndebele W, Ngwenya S (2013) Full-term abdominal extrauterine pregnancy complicated by post-operative ascites with successful outcome: a case report. J Med Case Report 7:10
Sentilhes L, Kayem G, Chandraharan E, Palacios-Jaraquemada J, Jauniaux E, FIGO placenta accreta diagnosis and management expert consensus panel; FIGO placenta accreta diagnosis and management expert consensus panel (2018) FIGO consensus guidelines on placenta accreta spectrum disorders: conservative management. Int J Gynaecol Obstet 140(3):291–298
Marcellin L, Delorme P, Bonnet MP, Grange G, Kayem G, Tsatsaris V et al (2018) Placenta percreta is associated with more frequent severe maternal morbidity than placenta accreta. Am J Obstet Gynecol 219(2):193.e1-193.e9
Sentilhes L, Deneux-Tharaux C, Seco A, Kayem G (2020) Conservative management versus cesarean hysterectomy for placenta accreta spectrum : the PACCRETA prospective study. Am J Obstet Gynecol 222(1):S3–S4
Garzon S, Raffaelli R, Montin U et al (2018) Primary hepatic pregnancy: report of a case treated with laparoscopic approach and review of the literature. Fertil Steril 110(5):925-931.e1
Rohilla M, Joshi B, Jain V et al (2018) Advanced abdominal pregnancy: a search for consensus. Review of literature along with case report. Arch Gynecol Obstet. https://doi.org/10.1007/s00404-018-4743-3
Funding
This study was supported by the Fundamental Research Funds of Central Public Welfare Scientific Institution of the Chinese Academy of Medical Science [2019PT320011].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YC, XL. The first draft of the manuscript was written by YC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Institutional Review Board (IRB) of Peking Union Medical College Hospital (PUMCH) has determined that this study is exempt from full IRB review based on the following reason: the study only involves the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, and these sources are publicly available or the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects (approval number: S-K1748).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Peng, P., Li, C. et al. Abdominal pregnancy: a case report and review of 17 cases. Arch Gynecol Obstet 307, 263–274 (2023). https://doi.org/10.1007/s00404-022-06570-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06570-9