Abstract
Purpose
Testicular stromal tumors are uncommon, although mostly benign. The purpose of this study is to assess the role of multi-parametric MRI in differentiating benign testicular stromal tumors from malignant testicular neoplasms (non-stromal and stromal).
Methods
A single-center retrospective study comparing benign stromal tumors (STs) to malignant testicular neoplasms (MTNs) was conducted. MR imaging assessment included tumor size, T2- and T1-weighted signal intensity, T2- and T1-weighted texture pattern, diffusion restriction, presence of hemorrhage and/or necrosis, and measurement of apparent diffusion coefficient and dynamic contrast enhancement (DCE). Inter-observer agreement was assessed using Cohen’s kappa and Bland–Altman and data were compared using independent t-tests or χ2. Receiver operating characteristic curve analysis was used to test models incorporating various imaging features.
Results
Radical orchiectomy and histopathology revealed 20 testicular neoplasms: seven STs (35%) and thirteen MTNs (65%). MTNs were significantly larger in size than STs (5.1 ± 2.36 cm vs. 1.27 ± 0.56 cm; p-value < 0.001). STs demonstrated more hypointense T2W signal (85.7% vs. 46.2%; p-value < 0.09), less T2W heterogeneous texture (14.3% vs. 61.5%; p-value < 0.04), and less diffusion restriction (16.7% vs. 83.3%; p-value < 0.01) in comparison to MTNs. STs demonstrated mainly homogenous post-contrast enhancement pattern (71.4% vs. 7.7%; p-value < 0.004), while MTNs showed primarily heterogeneous enhancement pattern (77% vs. 14.3%; p-value < 0.02). STs revealed greater corrected venous phase enhancement (STs: 0.59 ± 0.29; MTNs: 0.25 ± 0.25; p-value < 0.03). STs showed higher ADC values, though the difference was not statistically significant (p-value < 0.25). A model combining T2W, DWI, and DCE features showed the best overall diagnostic accuracy with area under ROC curve of 0.99 and confidence interval ranging from 0.94 to 1.
Conclusion
Multi-parametric MRI can potentially differentiate benign stromal tumors from malignant testicular neoplasms, which can help to avoid radical orchiectomy. However, future studies using larger sample sizes are needed to validate our results.
Similar content being viewed by others
Introduction
Testicular cancer accounts for approximately 1% of all malignant tumors in males and is the most common tumor encountered in young men aged 15–35 years [1,2,3,4]. The most common type of testicular carcinomas are germ cell tumors (GCTs), representing 95% of primary neoplasms split evenly between classic seminomas and non-seminomatous germ cell tumors [5]. Sex cord stromal tumors (STs) represent the remaining 5% of primary testicular tumors [6] and originate from the testicular interstitium, which is derived from mesenchymal cells. These tumors are relatively rare but they are mostly (> 90%) benign.
Imaging is essential for the diagnosis of testicular neoplasms. However, differentiation between various testicular malignancies on imaging remains challenging [1]. Magnetic resonance imaging (MRI) provides detailed morphological information and potential physiological correlation of tumor perfusion and structure that can be useful for local staging of testicular cancer [7,8,9]. Although high spatial resolution T2-weighted imaging (T2W) is integral for assessment of intra-testicular tumors, functional imaging techniques including diffusion-weighted (DWI) and dynamic contrast-enhanced (DCE) imaging are valuable sequences and should be included for evaluation of testicular cancer, but are yet to be fully explored [10,11,12,13,14,15].
Prior studies have shown that MRI may be a helpful tool in the evaluation and characterization of benign and malignant features of testicular tumors [1, 7]; however, few studies evaluated benign testicular STs [15, 16] which are a common cause of benign diagnosis after orchiectomy [17]. The pre-operative diagnosis of benign STs could prevent unnecessary radical orchiectomy and preserve fertility if the diagnosis is raised on imaging and confirmed intraoperatively at frozen section analysis [17,18,19,20,21,22]. The purpose of this preliminary study is to evaluate the diagnostic utility of multi-parametric magnetic resonance imaging (mp-MRI) to differentiate STs from malignant testicular neoplasms (MTNs), stromal and non-stromal.
Materials and methods
With institutional review board (IRB) approval, we identified all patients who were referred to the Radiology Department at our institution for clinically or sonographically indeterminate testicular mass with presurgical MRI and histopathological confirmation following radical orchiectomy, between April 2011 and September 2016. 20 male patients met the study inclusion criteria.
Magnetic resonance imaging
All patients underwent MRI evaluation using a 1.5-T clinical MRI scanner with a 16-channel surface coil (Magnetom Avanto, Siemens Healthcare, Malvern PA). A standard MR imaging protocol was performed in all cases, including T2W, T1W, DWI and DCE imaging (Table 1).
Magnetic resonance imaging data analysis
Two blinded radiologists with 10 and 15 years of experience in reporting abdominal MRI examinations independently assessed the qualitative and quantitative MRI findings. All examinations were reviewed using a standard electronic PACS (GE Centricity-RIS V. 4.2 plus).
Qualitative analysis
Two radiologists assessed the qualitative features: (a) T2W and T1W signal intensity (SI) relative to the normal testicular parenchyma was assessed using a three-point scale (1 = hyperintense, 2 = hypointense, 3 = isointense; (b) T2W and T1W texture pattern were assessed on T2W and T1W images as a binary outcome—Homogeneous (uniform SI) and heterogeneous (mixed areas of differing SI i.e., lesion containing a variable signal combination of hyperintensity, hypointensity, and isointensity); (c) Diffusion restriction (binary outcome where 1 = present [i.e., the tumor showed low signal on apparent diffusion coefficient (ADC) map with corresponding increasingly bright signal on trace DWI images with increasing b value] or 0 = absent [i.e., increased signal on ADC map or no increase in signal on trace DWI images with increasing b values]); (d) Tumor enhancement patterns (1 = homogeneous, 2 = heterogeneous, 3 = peripheral, or 4 = non-enhancing); (e). The DCE curves were documented as (Table 2): type-1 an incremental contrast pooling in the lesion, type-2 contrast enhancement with plateau and type-3 a washout curve [23]. (f) Hemorrhage defined as focal or diffuse areas of increased T1W SI within the lesion more than the normal testicular parenchyma and not representing fat on fat-suppressed images, represented as a binary outcome; 1 = present; 0 = absent.
Quantitative analysis
Two radiologists measured the quantitative features: (a) Lesion size was evaluated on T2W imaging in the three orthogonal planes. (b) ADC values were measured by placing a circular region of interest (ROI) encompassing at least two-thirds of the lesion at the center slice of the tumor avoiding the normal testicular parenchyma. (c) Semi-quantitative DCE curve analysis. DCE semi-quantitative curve analysis was performed using Syngo Somatom post-processing software (Version B-19; Siemens Healthcare, Malvern, PA). Pre- and post-gadolinium enhanced arterial, venous, delayed axial volumetric interpolated breath-hold examination (VIBE) images were uploaded on the mean curve application. For creating the DCE contrast curves, a ROI was carefully placed within each lesion matching (size and shape) with the ROI used for ADC analysis. Any large area of hemorrhage or necrosis was avoided from the ROI both during ADC value measurements and DCE curve analysis. ADC value and DCE enhancement pattern were also assessed for the normal testicular parenchyma by placing an ROI of the same size and shape on the contralateral normal testis at the same imaging level. Based on the SI measured for the lesion and the normal testicular parenchyma during each phase of contrast enhancement, a SI ratio was calculated using the formula (SI ratio = SI post/SI pre) and then corrected SI was calculated using the following formula (Corrected SI = SI lesion post-gadolinium − SI lesion pre-gadolinium/SI lesion pre-gadolinium) [24].
Assessment of MR imaging models for diagnosis of benign STs
Three diagnostic models were created from MRI findings and the accuracy of the models was assessed for the diagnosis of benign STs (Table 3). Diagnostic models in the study were created a priori based upon agreement by the two radiologists and the available published literature [9, 16, 25,26,27,28,29,30] to illustrate the incremental benefits of integrating T2W, DWI and DCE features for diagnosis. Germ cell tumors (both seminomatous and non-seminomatous) have been described to demonstrate T2W signal heterogeneity (not described with stromal—Leydig cell tumor) [9, 24]. Protocol A (T2-FSE alone) therefore used 5-point criteria to differentiate ST from MTNs (Table 3). Protocol B (combined assessment criteria using T2-FSE and DWI) was created based on the general agreement that malignant neoplasms demonstrate more marked diffusion restriction in comparison to the benign lesions. Although statistically significant differences in ADC values have been reported between Seminoma and non-seminomatous germ cell neoplasms, still the reported mean ADC ranges between 0.59 and 0.9 (10−3 mm2/s) [16]. Protocol C (assessment using a combination of T2-FSE, DWI and DCE curve) was used as the utility of DCE-MRI has been proved in the characterization of the various histological subtypes in malignancies such as breast, prostate, renal and ovaries [26, 29, 30]. Lesions with rapid contrast wash-in followed by a rapid washout (Type III time-intensity curve), have a high positive predictive value for the diagnosis of malignancy [26, 29, 30].
For each model, a subjective confidence score reflecting the probability of the presence of STs versus MTNs was assigned using the following five-point Likert scale (5-definitely MTNs; 4-probably MTNs; 3-possibly MTNs; 2-probable STs; and 1-definite STs). For DCE, a separate scoring criterion was added while reviewing lesions in model C based on the time-intensity curves and the degree of lesion enhancement in comparison to the background normal testis (Wash in and washout: Type III curve-5; Wash in and plateau: Type II-4; Progressive enhancement < than background-3; Mild to moderate progressive enhancement: Type I > than background-2; Marked progressive enhancement: Type I > than the background: 1). This was assessed to review the performance of single versus a combination of parameters in differentiating STs vs. MTNs (Table 3).
Histopathological findings
Histopathological outcome after orchiectomy was the standard of reference used in this study. The histologic sections of each case were reviewed in retrospect by one pathologist (IB) with more than 20 years of experience in testicular pathologies and diagnosis was confirmed as either benign STs or MTNs.
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago-USA) and MedCalc (MedCalc Software, Mariakerke, Belgium). For the agreement analysis of qualitative and quantitative findings between the 2 radiologists, Cohen’s Kappa and Bland–Altman analyses were performed, respectively. Comparisons between two groups were performed using either the independent sample t-tests or χ2 tests. Receiver operating characteristic (ROCs) curves and the corresponding areas under the ROC (AUCs) were estimated for the various models. The sensitivity, specificity, and area under the ROC curves were calculated. AUCs were compared among the model using ROC comparison function. A p-value of ≤ 0.05 was considered as significant level.
Results
Post-radical orchiectomy histopathology results revealed the presence of 20 testicular neoplasms. Ten cases (50%) were found to be GCTs including classic seminoma (n = 5; 25%); Embryonal carcinoma (n = 1; 5%); Immature teratoma (n = 1; 5%), Rhabdomyosarcoma and teratoma (n = 1; 5%); Choriocarcinoma (n = 1; 5%) and mixed germ cell tumor (n = 1; 5%). Three MTNs were non-GCTs and included one case of testicular lymphoma, carcinoma of unknown origin and malignant sex cord stromal tumor (5% each). Seven cases (35%), proved histologically to be benign STs including Leydig cell tumor (n = 5; 25%), Calcifying sertoli cell tumor (n = 1; 5%), and Granulosa cell tumor (n = 1; 5%). No significant age difference was observed between groups (STs: 30.9 ± 14.2 years; MTNs: 35.2 ± 15.3 years; p-value < 0.54).
Results for qualitative/subjective features are summarized in Table 2. STs were more hypointense on T2W (85.7% vs. 46.2%; p-value < 0.09), less heterogeneous (14.3% vs. 61.5%; p-value < 0.04), and less diffusion restricting (16.7% vs. 83.3%; p-value < 0.01) than MTNs (Table 2).
STs (Fig. 1) demonstrated predominantly homogenous appearance on post-contrast images (71.4% vs. 7.7%; p-value < 0.004), while MTNs showed predominantly heterogeneous pattern (77% vs. 14.3%; p-value < 0.02) (Figs. 2, 3, 4). Inter-observer agreement for qualitative findings between the two readers was 0.92 to 1.
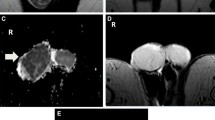
A 45-year-old male with right testicular benign Leydig cell tumor. Ultrasound examination to rule out varicocele (a) demonstrates an impalpable 8 mm hypoechoic right intra-testicular nodule with internal vascularity (arrow), suggesting its solid nature. Axial (b) and coronal (c) T2W images show a well-circumscribed mainly hypointense intra-testicular lesion (arrow) against a bright normal testicular parenchyma signal. The lesion demonstrates no diffusion restriction appearing dark (arrow) on both high b value DWI (d) and corresponding ADC map (e) with mean ADC value of 1064 × 10−3 mm2/s. Axial unenhanced (f) and dynamic post-contrast (g–i) T1 fat-saturated spoiled gradient-echo images show marked lesion enhancement on the venous phase (arrow on h, i), in comparison to the background testicular parenchyma. Time-intensity enhancement curve (j) demonstrates marked tumor enhancement (Type-1) compared to the background parenchyma (DCE scoring criteria 1)
A 27-year-old male with right testicular seminoma. Axial (a) and sagittal (b) T2W images demonstrate nearly complete replacement of the right testis by an inhomogeneous mass with peripheral irregular rind of soft tissue mainly of low T2 signal (white arrows) and central necrosis appearing bright on T2 (dotted arrow). The oval ROI (circle, a) demonstrates how ADC value and DCE curves were obtained avoiding the area of necrosis. Axial b-800 DWI (c) and corresponding ADC map (f) show significant diffusion restriction (arrows), with mean ADC value of 1126 × 10−3 mm2/s. Coronal corresponding T1W images pre- (d) and post-contrast VIBE (e) show only mild tumor enhancement with reticular areas (white arrow). Note that central necrosis area is not enhancing (black arrows). Time-intensity enhancement curve (g) demonstrates slightly marked tumor enhancement than the background parenchyma in early phases, which homogenizes with the background on subsequent delayed phases
A 40-year-old male with left testicular malignant sex cord stromal tumor (unclassified type). Sagittal (a), axial (b), and coronal (c) T2W images show large inhomogeneous mass nearly completely replacing the left testis (long white arrows). Multiple foci of markedly high T2 signal within the mass indicating necrosis (short white arrows). Coronal T1W post-contrast image (e) shows irregular area of tumor enhancement (arrows). Curvilinear hyperintense signal (dashed arrow) along the cranial aspect of the mass on T1W pre-contrast image (d) corresponds to the hypointense signal on T2W image (dashed arrow, c) signifying early subacute hemorrhage. Note the susceptibility effects of the blood products (dashed arrow) with an intensely bright signal on b-800 DW image (f) and intensely low signal on the corresponding ADC map (g). Tumor diffusion restriction is also noted (white arrows, f and g). Large ipsilateral hydrocele also is seen (*)
A 68-year-old male with left testicular large B-cell lymphoma. Coronal (a), sagittal (b), and axial (c) T2W images show a hypointense testicular mass (solid arrow) with contiguous epididymal head involvement (dashed arrow). The lesion presents solid enhancement with linear and reticular areas on coronal post-contrast T1W VIBE image (d). Axial DWI b-800 image (f) and corresponding ADC map (g) demonstrate marked tumor restricted diffusion (arrows) when compared to the background testicular parenchyma with mean tumor ADC value of 623 × 10−3 mm2/s. Note the presence of extensive retroperitoneal lymph nodal mass (*) on coronal abdomen post-contrast T1 image (e). Time-intensity enhancement curve (h) demonstrates slightly marked tumor enhancement than the background parenchyma during the venous and delayed phases
The results for quantitative measurements are summarized in Table 4. A significantly larger size was observed in MTNs than STs (5.1 ± 2.36 cm vs. 1.27 ± 0.56 cm; p-value < 0.001). ADC values were higher in STs (960 ± 259 × 10−3mm2/s) as compared to MTNs (793 ± 306 × 10−3mm2/s), although the difference was not significant (p-value < 0.27). Using corrected DCE SI; STs were more enhancing on venous phase (STs: 0.59 ± 0.29; MTNs: 0.25 ± 0.25; p-value < 0.03) (Fig. 5). Bland–Altman showed a low mean difference between measurements for quantitative variables measured.
In ROC analysis (Fig. 6) to assess the performance of different sequences alone or in combination models, there was an increasing trend towards higher accuracy in model A, B and C (area under curves [AUCs] = 0.90, 0.95, 0.99; confidence intervals 0.75–1, 0.85–1, 0.94–1), respectively (Table 5). Although there was no significantly different accuracy comparing models, model C (which incorporated T2W, DWI, and DCE features) showed a trend towards a significant difference compared to model A (p-value < 0.06). There were increasing trends towards improved sensitivity, specificity and accuracy from model A to B to C and positive, negative predictive values were better for model C than model A and B (Table 5).
Discussion
Our study results indicate that smaller tumor size, relatively more T2W hypointense signal, less T2W heterogeneous texture, homogeneous enhancement, significantly increased and progressive enhancement during the venous phase and insignificant diffusion restriction may differentiate between benign stromal tumors and malignant testicular neoplasms. However, in qualitative assessment, we found a significant overlap between STs and MTNs (T2W texture and presence of hemorrhage) (Table 2). It is the insignificant diffusion restriction and homogenous enhancement (in the venous phase) that were able to differentiate between benign STs and MTNs. Similar to prior publications in literature, we observed that larger seminomas has an increased chance of hemorrhage; however, larger foci of hemorrhage and necrosis/cystic changes were more common in non-seminomatous GCTs [7]. Additionally, combining sequences showed overall increase accuracy for differentiating benign stromal tumors from malignant testicular neoplasms.
The current study suggests that hypointense T2 signal intensity and less T2W heterogeneous texture are important morphological features differentiating STs from MTNs and can be considered as a differentiating imaging biomarker of these pathologies. Manganaro et al. had published similar observations previously between Leydig cell tumors and MTNs [31]. However, we also observed that T2W tumor heterogeneity is one of the important differentiating morphological features that have not been reported in their study. In our opinion, this may be related to tumor necrosis and hemorrhage, which is a feature of malignant tumors. Our results are also in line with the smaller size encountered for STs as observed in Manganaro et al. study [31].
On semi-quantitative MRI evaluations between groups (such as DWI and DCE), lower ADC values were observed in MTNs; however, the difference was statistically insignificant. Our findings on ADC are similar to the previously reported results by Talakic et al. which showed insignificantly higher ADC values in benign tumors [32]. In our study, we also observed less restriction diffusion in STs than MTNs, which could be an important differentiating feature of STs from MTNs.
In Manganaro et al. study [31], STs showed more marked progressive enhancement on DCE images than MTNs, weak progressive wash-in and prolonged washout in Leydig cell tumors. While this was not encountered in our cohort, the differences could be attributed to variations in the DCE protocol between the two studies where the authors used consecutive phases of 20 s each to create wash-in and washout curves. In our institution, we don’t routinely follow contrast washout that long which is possibly the reason for not getting similar delayed washout patterns.
Our study did not find time-intensity curves helpful in differentiating STs from MTNs with overlapping time-intensity curves between the two categories, unlike earlier reports by Tsili et al. [30] where all GCTs demonstrated type-3 time-intensity enhancement curve (n = 26). This also could be due to the differences in the DCE protocol used. However, our study was able to differentiate between the STs and MTNs using the corrected DCE SI during the venous phase using the routine multi-phasic post-contrast sequence (without DCE protocol modifications).
A corrected DCE SI assessment demonstrated statistically significant differences between the two groups in the venous phase with a significantly higher enhancement of STs than MTNs suggesting a role of DCE in differentiating between ST and MTNs in clinically or Ultrasound indeterminate testicular lesions.
The assessment of different sequences either alone or in combinations (models A, B, and C) showed that integration of T2W, DWI and DCE (model C) has an incremental trend towards improved accuracy in differentiating STs from MTNs. Similar to our study results, Serra et al. reported an overall accuracy of 91% for MR imaging in differentiating benign from malignant testicular lesions in a study of 29 testicular masses, following inconclusive clinical and sonographic evaluation [33].
Manganaro et al. reported a sensitivity and specificity of 89.47% and 95.65%, respectively, with scrotal MRI in diagnosing Leydig cell tumors and a sensitivity and specificity of 95.65% and 80.95%, respectively, for malignant lesions [31]. Our study, combining both the qualitative and quantitative MRI parameters, had a slightly better sensitivity (92%), specificity (100%) and overall accuracy (95%) for differentiating STs from MTNs. Our findings were not limited to the diagnosis of Leydig cell tumors only and rather included other benign stromal tumors also.
Our study though has certain limitations: (1) It is a single institutional retrospective study with a small sample size. Having larger sample size may lead to a greater power to see the significant difference between the groups in the future. (2) STs represented 35% of our total cases, which could be due to direct surgical management of cases with malignant tumor findings on clinical and ultrasound exams. (3) DCE analysis, in our study, was performed semi-quantitatively using routinely acquired pre- and 4 phase post-gadolinium enhanced imaging. The temporal resolution in our study limits our DCE analysis; however, the contrast enhancement parameters used are similar to the routinely performed abdominal/pelvic MRI exams. Future studies are required in a larger cohort to validate our results before they can be applied clinically to prompt a surgeon to determine the diagnosis of testicular tumors using frozen section analysis at the surgery, which can potentially prevent unnecessary orchiectomy in a subset of patients.
Conclusion
Our preliminary results suggest that mp-MRI may pre-operatively differentiate between benign sex cord stromal tumors and malignant testicular neoplasms. A model incorporating significant features achieved a high level of accuracy; however, it requires further external validation to be incorporated into clinical practice.
References
Coursey Moreno C, Small WC, Camacho JC, Master V, Kokabi N, Lewis M, Hartman M, Mittal PK (2015) Testicular tumors: what radiologists need to know–differential diagnosis, staging, and management. Radiographics 35 (2):400-415. https://doi.org/10.1148/rg.352140097
Einhorn LH (2002) Curing metastatic testicular cancer. Proceedings of the National Academy of Sciences 99 (7):4592-4595. https://doi.org/10.1073/pnas.072067999
Garner MJ, Turner MC, Ghadirian P, Krewski D (2005) Epidemiology of testicular cancer: an overview. Int J Cancer 116 (3):331-339. https://doi.org/10.1002/ijc.21032
Liu S, Wen SW, Mao Y, Mery L, Rouleau J (1999) Birth cohort effects underlying the increasing testicular cancer incidence in Canada. Can J Public Health 90 (3):176-180
Ulbright TM (2005) Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod Pathol 18 Suppl 2:S61-79. https://doi.org/10.1038/modpathol.3800310
Krag Jacobsen G, Barlebo H, Olsen J, Schultz HP, Starklint H, Sogaard H, Vaeth M (1984) Testicular germ cell tumours in Denmark 1976-1980. Pathology of 1058 consecutive cases. Acta Radiol Oncol 23 (4):239-247. https://doi.org/10.3109/02841868409136019
Tsili AC, Argyropoulou MI, Giannakis D, Sofikitis N, Tsampoulas K (2010) MRI in the characterization and local staging of testicular neoplasms. AJR Am J Roentgenol 194 (3):682-689. https://doi.org/10.2214/AJR.09.3256
Mohrs OK, Thoms H, Egner T, Brunier A, Eiers M, Kauczor HU, Hallscheidt P (2012) MRI of patients with suspected scrotal or testicular lesions: diagnostic value in daily practice. AJR Am J Roentgenol 199 (3):609-615. https://doi.org/10.2214/AJR.11.7349
Tsili AC, Tsampoulas C, Giannakopoulos X, Stefanou D, Alamanos Y, Sofikitis N, Efremidis SC (2007) MRI in the histologic characterization of testicular neoplasms. AJR Am J Roentgenol 189 (6):W331-337. https://doi.org/10.2214/AJR.07.2267
Tsili AC, Argyropoulou MI, Giannakis D, Tsampalas S, Sofikitis N, Tsampoulas K (2012) Diffusion-weighted MR imaging of normal and abnormal scrotum: preliminary results. Asian J Androl 14 (4):649-654. https://doi.org/10.1038/aja.2011.172
Watanabe Y, Dohke M, Ohkubo K, Ishimori T, Amoh Y, Okumura A, Oda K, Hayashi T, Dodo Y, Arai Y (2000) Scrotal disorders: evaluation of testicular enhancement patterns at dynamic contrast-enhanced subtraction MR imaging. Radiology 217 (1):219-227. https://doi.org/10.1148/radiology.217.1.r00oc41219
Algebally AM, Tantawy HI, Yousef RR, Szmigielski W, Darweesh A (2015) Advantage of Adding Diffusion Weighted Imaging to Routine MRI Examinations in the Diagnostics of Scrotal Lesions. Pol J Radiol 80:442-449. https://doi.org/10.12659/PJR.894399
Reinges MHT, Kaiser WA, Miersch WD, Vogel J, Reiser M (1995) Dynamic MRI of benign and malignant testicular lesions: preliminary observations. European Radiology 5 (6). https://doi.org/10.1007/bf00190927
Tsili AC, Bertolotto M, Turgut AT, Dogra V, Freeman S, Rocher L, Belfield J, Studniarek M, Ntorkou A, Derchi LE, Oyen R, Ramchandani P, Secil M, Richenberg J (2018) MRI of the scrotum: Recommendations of the ESUR Scrotal and Penile Imaging Working Group. Eur Radiol 28 (1):31-43. https://doi.org/10.1007/s00330-017-4944-3
El Sanharawi I, Correas JM, Glas L, Ferlicot S, Izard V, Ducot B, Bellin MF, Benoit G, Rocher L (2016) Non-palpable incidentally found testicular tumors: Differentiation between benign, malignant, and burned-out tumors using dynamic contrast-enhanced MRI. Eur J Radiol 85 (11):2072-2082. https://doi.org/10.1016/j.ejrad.2016.09.021
Tsitouridis I, Maskalidis C, Panagiotidou D, Kariki EP (2014) Eleven patients with testicular leydig cell tumors: clinical, imaging, and pathologic correlation. J Ultrasound Med 33 (10):1855-1864. https://doi.org/10.7863/ultra.33.10.1855
Giannarini G, Dieckmann KP, Albers P, Heidenreich A, Pizzocaro G (2010) Organ-sparing surgery for adult testicular tumours: a systematic review of the literature. Eur Urol 57 (5):780-790. https://doi.org/10.1016/j.eururo.2010.01.014
Giannarini G, Mogorovich A, Menchini Fabris F, Morelli G, De Maria M, Manassero F, Loggini B, Pingitore R, Cavazzana A, Selli C (2007) Long-term followup after elective testis sparing surgery for Leydig cell tumors: a single center experience. J Urol 178 (3 Pt 1):872-876; quiz 1129. https://doi.org/10.1016/j.juro.2007.05.077
Loeser A, Vergho DC, Katzenberger T, Brix D, Kocot A, Spahn M, Gerharz EW, Riedmiller H (2009) Testis-sparing surgery versus radical orchiectomy in patients with Leydig cell tumors. Urology 74 (2):370-372. https://doi.org/10.1016/j.urology.2009.03.014
Suardi N, Strada E, Colombo R, Freschi M, Salonia A, Lania C, Cestari A, Carmignani L, Guazzoni G, Rigatti P, Montorsi F (2009) Leydig cell tumour of the testis: presentation, therapy, long-term follow-up and the role of organ-sparing surgery in a single-institution experience. BJU Int 103 (2):197-200. https://doi.org/10.1111/j.1464-410X.2008.08016.x
Matei DV, Vartolomei MD, Renne G, Tringali VML, Russo A, Bianchi R, Cozzi G, Bottero D, Musi G, Mazzarol G, Ferro M, de Cobelli O (2017) Reliability of Frozen Section Examination in a Large Cohort of Testicular Masses: What Did We Learn? Clin Genitourin Cancer 15 (4):e689-e696. https://doi.org/10.1016/j.clgc.2017.01.012
Heidenreich A, Paffenholz P, Nestler T, Pfister D (2019) European Association of Urology Guidelines on Testis Cancer: Important Take Home Messages. Eur Urol Focus 5 (5):742-744. https://doi.org/10.1016/j.euf.2019.08.002
Hansford BG, Peng Y, Jiang Y, Vannier MW, Antic T, Thomas S, McCann S, Oto A (2015) Dynamic Contrast-enhanced MR Imaging Curve-type Analysis: Is It Helpful in the Differentiation of Prostate Cancer from Healthy Peripheral Zone? Radiology 275 (2):448-457. https://doi.org/10.1148/radiol.14140847
Shaida N, Berman LH (2012) Percutaneous testicular biopsy for indeterminate testicular lesions. Br J Radiol 85 Spec No 1:S54-58. https://doi.org/10.1259/bjr/30496032
Sun MR, Ngo L, Genega EM, Atkins MB, Finn ME, Rofsky NM, Pedrosa I (2009) Renal cell carcinoma: dynamic contrast-enhanced MR imaging for differentiation of tumor subtypes–correlation with pathologic findings. Radiology 250 (3):793-802. https://doi.org/10.1148/radiol.2503080995
Koo HR, Cho N, Song IC, Kim H, Chang JM, Yi A, Yun BL, Moon WK (2012) Correlation of perfusion parameters on dynamic contrast-enhanced MRI with prognostic factors and subtypes of breast cancers. J Magn Reson Imaging 36 (1):145-151. https://doi.org/10.1002/jmri.23635
Thomassin-Naggara I, Bazot M, Darai E, Callard P, Thomassin J, Cuenod CA (2008) Epithelial ovarian tumors: value of dynamic contrast-enhanced MR imaging and correlation with tumor angiogenesis. Radiology 248 (1):148-159. https://doi.org/10.1148/radiol.2481071120
Tsili AC, Argyropoulou MI, Astrakas LG, Ntoulia EA, Giannakis D, Sofikitis N, Tsampoulas K (2013) Dynamic contrast-enhanced subtraction MRI for characterizing intratesticular mass lesions. AJR Am J Roentgenol 200 (3):578-585. https://doi.org/10.2214/AJR.12.9064
Cornelis F, Tricaud E, Lasserre AS, Petitpierre F, Bernhard JC, Le Bras Y, Yacoub M, Bouzgarrou M, Ravaud A, Grenier N (2014) Routinely performed multiparametric magnetic resonance imaging helps to differentiate common subtypes of renal tumours. Eur Radiol 24 (5):1068-1080. https://doi.org/10.1007/s00330-014-3107-z
Tsili AC, Sylakos A, Ntorkou A, Stavrou S, Astrakas LG, Sofikitis N, Argyropoulou MI (2015) Apparent diffusion coefficient values and dynamic contrast enhancement patterns in differentiating seminomas from nonseminomatous testicular neoplasms. Eur J Radiol 84 (7):1219-1226. https://doi.org/10.1016/j.ejrad.2015.04.004
Manganaro L, Vinci V, Pozza C, Saldari M, Gianfrilli D, Pofi R, Bernardo S, Cantisani V, Lenzi A, Scialpi M, Catalano C, Isidori AM (2015) A prospective study on contrast-enhanced magnetic resonance imaging of testicular lesions: distinctive features of Leydig cell tumours. Eur Radiol 25 (12):3586-3595. https://doi.org/10.1007/s00330-015-3766-4
Talakic E TS SH, Manfred T (2014) Diffusion Weighted Imaging of Testicular Tumors – Is there a Value for Differentiation between Benign and Malignant?. Radiological Society of North America
Serra AD, Hricak H, Coakley FV, Kim B, Dudley A, Morey A, Tschumper B, Carroll PR (1998) Inconclusive Clinical and Ultrasound Evaluation of the Scrotum: Impact of Magnetic Resonance Imaging on Patient Management and Cost. Urology 51 (6):1018-1021. https://doi.org/10.1016/s0090-4295(98)00097-1
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khanna, M., Abualruz, A.R., Yadav, S.K. et al. Diagnostic performance of multi-parametric MRI to differentiate benign sex cord stromal tumors from malignant (non-stromal and stromal) testicular neoplasms. Abdom Radiol 46, 319–330 (2021). https://doi.org/10.1007/s00261-020-02621-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02621-4