Practice Essentials
First described in 1982 by Hassoun et al, central neurocytoma (CN) is a rare tumor of neuroglial origin. CN is generally regarded as a benign neoplasm with a favorable prognosis and affects mainly young adults, with the mean age of presentation being 29 years (age range, 8 days to 67 years). Central neurocytoma is typically located in the lateral ventricles, near the foramen of Monro, with a characteristic attachment to the septum pellucidum. Central neurocytomas are rare, constituting only 0.25-0.50% of all intracranial tumors. However, they are the most common primary intraventricular tumors in adults. [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]
According to the World Health Organization classification, central neurocytoma is a benign grade II tumor with a good prognosis. [11, 2, 3] In a study of 40 patients with typical or atypical CN, the typical CN 2-, 3-, and 5-year progression-free survival rates were 100%, 100%, and 92.3%, and those for the atypical group were 93.8%, 78.1%, 65.1%, respectively. [12] In a study of characteristics of patients with CN according to the National Cancer Database (NCDB), by Dutta et al, of 223,404 patients with brain tumors, 868 patients were diagnosed with neurocytoma (0.4%, or approximately 75 patients annually), and the 5-year survival rate was 89%. [5]
On CT scans, CN is isoattenuating or slightly hyperattenuating and is a well-demarcated, lobulated mass with moderate to strong heterogeneous contrast enhancement. On MRI, CN is typically heterogeneous on all sequences, but it is isointense to cerebral cortex on T1-weighted images and isointense-to-hyperintense on T2-weighted images.
Extraventricular neurocytomas have been described. They can be situated in the cerebral hemispheres, pons, cerebellum, spinal cord, and retina. They behave in a similar fashion to their intraventricular counterparts. [13]
(See the central neurocytoma images below.)
 Axial GRE image at the level of the lateral ventricles demonstrates a small midline hypointense mass attached to the septum pellucidum. The hypointensity reflects calcification and/or hemosiderin deposition.
Axial GRE image at the level of the lateral ventricles demonstrates a small midline hypointense mass attached to the septum pellucidum. The hypointensity reflects calcification and/or hemosiderin deposition.
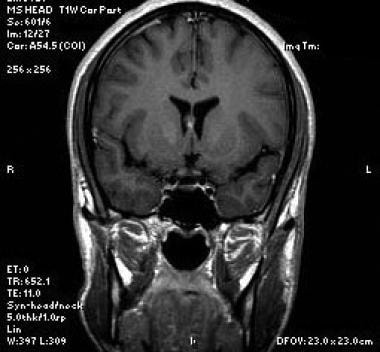 Coronal T1-weighted sequence at the level of the frontal horns of the lateral ventricles nicely depicts the mass intimately related to the septum pellucidum.
Coronal T1-weighted sequence at the level of the frontal horns of the lateral ventricles nicely depicts the mass intimately related to the septum pellucidum.
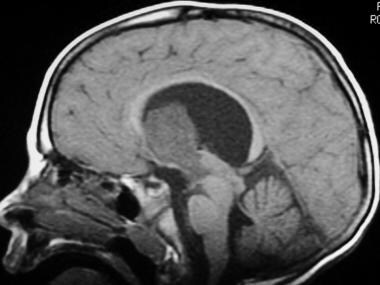 Sagittal midline T1-weighted sequence shows a large homogeneous mass, hypointense to brain parenchyma, exophytically extending into the third ventricle. Note the enlarged lateral ventricles, with mass effect on the corpus callosum.
Sagittal midline T1-weighted sequence shows a large homogeneous mass, hypointense to brain parenchyma, exophytically extending into the third ventricle. Note the enlarged lateral ventricles, with mass effect on the corpus callosum.
The diagnosis and management of CN remains controversial because most clinical series are small. The most important therapeutic modality is surgery, and a safe maximal resection confers the best long-term outcome. Inoperable tumors have been treated with a variety of chemotherapy agents. [14]
Clinical presentation and pathophysiology
Because of the intraventricular location of the vast majority of CNs, the most common presenting signs and symptoms are related to hyrocephalus from obstruction of CSF flow in the ventricular system. Patients may present with acute symptoms related to sudden development of ventricular obstruction and elevated intracranial pressures, or symptoms may be more insidious. Headache, mental status changes, and seizures may occur. [10]
Schild et al analyzed 27 patients with central neurocytomas regarding their presenting symptoms, and 93% of patients complained of headaches, 37% had visual changes, and 30% experienced nausea and vomiting at presentation. [15] Most of these symptoms were attributed to increased intracranial pressure secondary to obstructive hydrocephalus. Cases with more acute presentation due to hemorrhage into the tumor have also been well described. In a study by Wang et al of 27 patients, 21 presented with headache and 6 with vomiting. [16]
On visual inspection, central neurocytomas appear as soft, tan-colored, well-demarcated masses. The consistency can vary from soft to gritty, depending on the degree of calcification. The tumor usually does not have a large zone of attachment of surrounding brain tissue. However, there may be attachment to the surrounding ventricular surfaces, rendering gross total resection difficult or impossible.
Histologically, a central neurocytoma is characterized by a small, uniform neoplastic cell population with features of neuronal differentiation. [17] Perivascular arrangement of neurophils may mimic the perivascular pseudorosettes of ependymoma. Artificial perinuclear halo formation creates a remarkable likeness to oligodendroglioma. CN is immunoreactive to the neuronal marker proteins neuron-specific enolase (NSE) and synaptophysin. [18, 19]
Studies have shown that neurocytoma cells exhibit both neural and glial phenotypes and have the properties reminiscent of precursor cells derived from the periventricular matrix. For this reason, this tumor can be considered as a glial neuronal tumor with predominantly neurocytic differentiation. It remains controversial as to whether CN arises from cells committed to neuronal phenotype or from bipotential cells in the periventricular matrix.
Prognosis
Central neurocytoma is histologically benign and has an excellent prognosis. [20, 21, 22, 23, 2, 5] Lateral ventricular tumors can be resected via a transcortical or transcallosal approach. In the Wang study of 27 patients with central neurocytoma, there were no recurrences in the 17 patients who underwent gross total resection with no adjuvant therapy. [16] In contrast, the less common atypical neurocytoma is associated with increased proliferative activity and a high rate of recurrence. Craniospinal dissemination has been described. [24, 25]
The monoclonal antibody MIB-1 labeling index (LI) appears to be the best predictor of proliferative potential and clinical outcome of CN. MIB-1 LI seems to make it possible to distinguish between CN and atypical neurocytoma, characterized by a higher incidence of recurrence and an MIB-1 index greater than 2%. Soylemezoglu et al [26] concluded that a MIB-1 LI of 2% might be critical in determining recurrence. After a follow-up of 150 months, they observed that patients with an MIB-1 LI less than 2% had a relapse rate of 22%, compared with 63% when the level was greater than 2%. [27, 28, 29]
Stereotactic radiosurgery can be an effective and safe alternative therapy for recurrent or residual central neurocytoma. [30, 25]
Computed Tomography
On CT scans, central neurocytoma (CN) is isoattenuating or slightly hyperattenuating and is a well-demarcated, lobulated mass with moderate to strong heterogeneous contrast enhancement. Calcification is seen in about 50% of cases and is usually clumped, amorphous, or globular. [31, 32] Cystic changes have also been reported. [16]
Tumor location is a key imaging finding. CN is typically centered on the midline near the foramen of Monro with a broad-based attachment to the septum pellucidum. The imaging differential diagnosis consists of other intraventricular tumors that occur in young adults, including, but not limited to, oligodendroglioma, subependymal giant cell astrocytoma, low-grade or pilocytic astrocytoma, subependymoma, and ependymoma. [33, 34]
Magnetic Resonance Imaging
On MRI, central neurocytoma (CN) is typically heterogeneous on all sequences, but it is isointense to cerebral cortex on T1-weighted images and isointense-to-hyperintense on T2-weighted images. Areas of low signal intensity or absent signal on both T1- and T2-weighted images can represent calcification, cyst, hemorrhage, and tumor vessels. Most tumors show some degree on enhancement. [16, 35, 36, 37]
Extraventricular neurocytomas are typically well-demarcated masses, may have cystic components in up to 50%, enhance variably, and may or may not be associated cerebral edema. [13, 38]
In one MRI review of 12 patients, spicules were identified at the tumor periphery abutting the walls of the lateral ventricles. These spicules were formed by walls of multiple cysts of medium size. There was adjacent ventricular wall undulation. This combination of spicules and undulation lead to a “scalloping” appearance. Recognition of this appearance was thought to be a diagnostic sign of central neurocytoma. [39]
MR spectroscopy can be used to differentiate central neurocytomas from other intraventricular tumors such as meningioma. Specifically, compounds such as glycine and alanine may show significant differences between central neurocytomas and meningiomas. [40, 41]
Using dynamic susceptibility contrast-enhanced MR perfusion to assess intraventricular tumors, central neurocytomas reveal intermediate vascularity (mean relative cerebral blood volume [rCBV] between 2 and 3), compared with highly vascularized tumors such as papillomas, meningiomas, and renal carcinoma metastases, which have a mean rCBV of greater than 3, and poorly vascularized tumors such as ependymomas and subependymomas, showing a mean rCBV of less than 2. [42, 43]
(See the images below of central neurocytomas on MRI.)
 Axial GRE image at the level of the lateral ventricles demonstrates a small midline hypointense mass attached to the septum pellucidum. The hypointensity reflects calcification and/or hemosiderin deposition.
Axial GRE image at the level of the lateral ventricles demonstrates a small midline hypointense mass attached to the septum pellucidum. The hypointensity reflects calcification and/or hemosiderin deposition.
 Coronal T1-weighted sequence at the level of the frontal horns of the lateral ventricles nicely depicts the mass intimately related to the septum pellucidum.
Coronal T1-weighted sequence at the level of the frontal horns of the lateral ventricles nicely depicts the mass intimately related to the septum pellucidum.
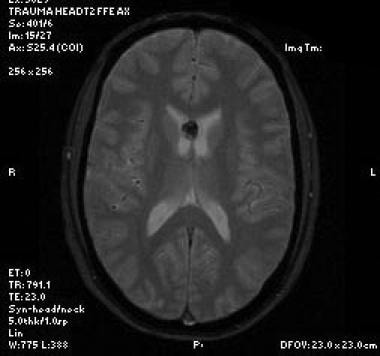
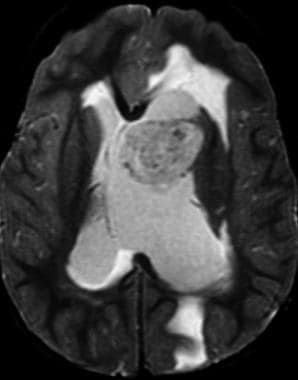 Axial T2-weighted sequence shows findings of a parasagittal intraventricular mass with obstructive hydrocephalus. Note the internal heterogeneity of the mass, a common finding on T2-weighted MRI.
Axial T2-weighted sequence shows findings of a parasagittal intraventricular mass with obstructive hydrocephalus. Note the internal heterogeneity of the mass, a common finding on T2-weighted MRI.
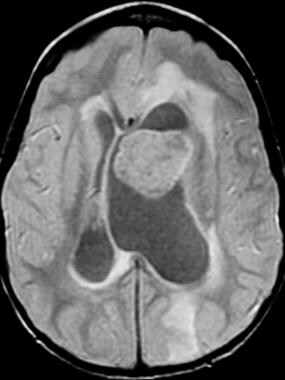 Axial proton-density-weighted sequence at the level of the lateral ventricles demonstrates the large mass. There is obstructive hydrocephalus, as well as associated periventricular signal abnormality.
Axial proton-density-weighted sequence at the level of the lateral ventricles demonstrates the large mass. There is obstructive hydrocephalus, as well as associated periventricular signal abnormality.
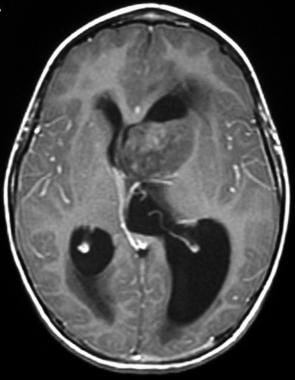 The T1-weighted sequence demonstrates partial internal heterogeneous enhancement of the mass following intravenous gadolinium administration. Note the local mass effect and asymmetric obstruction of the lateral ventricles.
The T1-weighted sequence demonstrates partial internal heterogeneous enhancement of the mass following intravenous gadolinium administration. Note the local mass effect and asymmetric obstruction of the lateral ventricles.
 Sagittal midline T1-weighted sequence shows a large homogeneous mass, hypointense to brain parenchyma, exophytically extending into the third ventricle. Note the enlarged lateral ventricles, with mass effect on the corpus callosum.
Sagittal midline T1-weighted sequence shows a large homogeneous mass, hypointense to brain parenchyma, exophytically extending into the third ventricle. Note the enlarged lateral ventricles, with mass effect on the corpus callosum.
Small cystic areas are typical on T2-weighted images. CN typically shows moderate to strong heterogeneous enhancement after gadolinium administration. However, some CNs do not have appreciable enhancement and may thus mimic the appearance of subependymoma.
The MR spectroscopic findings of CN have been reported in the literature. CN may show a characteristic metabolic profile on proton MR spectroscopy, consisting of high choline and low NAA peaks plus glycine and/or alanine peaks. [44, 45, 46, 47, 48, 49, 50, 51]
Chuang et al reported 3T MR spectroscopy findings in cases of CN confirmed by immunohistochemical stains. [44] Using a TE of 135 ms, they concluded that a glycine peak at 3.35 ppm is highly suggestive of CN but not diagnostic. In the same study, Chuang confirmed the presence of alanine in CN.
Krishnamoorthy et al demonstrated an alanine peak in 3 histopathologically confirmed cases of CN. [50]
Alanine occurs as an inverted doublet at 1.5 ppm using intermediate TE values (135-144 ms). Apart from CN, alanine is characteristically observed in meningiomas. The presence of an alanine peak signifies a poor prognosis in CN . [44, 52, 53]
-
Axial GRE image at the level of the lateral ventricles demonstrates a small midline hypointense mass attached to the septum pellucidum. The hypointensity reflects calcification and/or hemosiderin deposition.
-
Coronal T1-weighted sequence at the level of the frontal horns of the lateral ventricles nicely depicts the mass intimately related to the septum pellucidum.
-
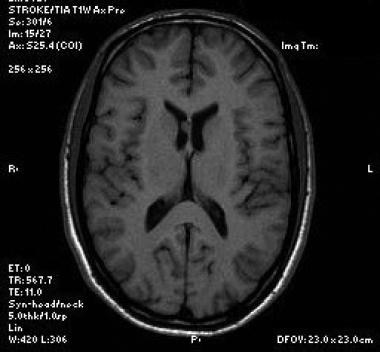
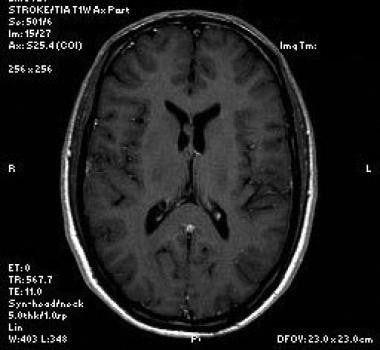
Axial T1-weighted sequence shows the mass to be isotense to brain parenchyma.
-
Axial T2-weighted sequence shows findings of a parasagittal intraventricular mass with obstructive hydrocephalus. Note the internal heterogeneity of the mass, a common finding on T2-weighted MRI.
-
Post-gadolinium T1-weighted sequence shows the mass does not enhance.
-
Axial proton-density-weighted sequence at the level of the lateral ventricles demonstrates the large mass. There is obstructive hydrocephalus, as well as associated periventricular signal abnormality.
-
The T1-weighted sequence demonstrates partial internal heterogeneous enhancement of the mass following intravenous gadolinium administration. Note the local mass effect and asymmetric obstruction of the lateral ventricles.
-
Sagittal midline T1-weighted sequence shows a large homogeneous mass, hypointense to brain parenchyma, exophytically extending into the third ventricle. Note the enlarged lateral ventricles, with mass effect on the corpus callosum.