Practice Essentials
Cushing syndrome, first described by Harvey in 1912, refers to signs and symptoms caused by excess free plasma glucocorticoids. Excess glucocorticoids can arise from increased endogenous production or prolonged exposure to exogenous use of glucocorticoid products. While endogenous Cushing syndrome is a rare disease, iatrogenic (drug-related or exogenous) Cushing syndrome from glucocorticoid products is commonly seen in clinical practice. This article will focuses on iatrogenic, or drug-related, Cushing syndrome. [1]
The diagnosis of Cushing syndrome requires demonstration of an inappropriately high level of cortisol in the serum or urine. Drugs that have been reported to result in hypercortisolism are glucocorticoids, megestrol acetate, and herbal preparations that contain glucocorticoids.
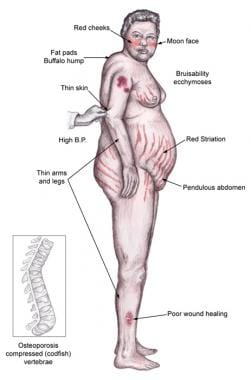
Individuals with Cushing syndrome can develop moon facies, facial plethora, supraclavicular fat pads, buffalo hump, truncal obesity, and purple striae, as shown in the image below.
Individuals often experience proximal muscle weakness, easy bruising, weight gain, hirsutism, and, in children, growth retardation. Hypertension, osteopenia, diabetes mellitus, and impaired immune function may also occur.
Signs and symptoms of Cushing syndrome
Patients may have increased adipose tissue in the face (moon facies), upper back at the base of neck (buffalo hump), and above the clavicles (supraclavicular fat pads).
Central obesity is characterized by increased adipose tissue in the mediastinum and peritoneum and an increased waist-to-hip ratio of greater than 1 in men and higher than 0.8 in women.
With regard to Cushing syndrome’s effects on the skin:
-
Facial plethora may be present, especially over the cheeks
-
Violaceous striae, often wider than 0.5 cm, are observed most commonly over the abdomen, buttocks, lower back, upper thighs, upper arms, and breasts
-
Ecchymoses may be present
-
Patients may have telangiectasias and purpura
-
Cutaneous atrophy with exposure of subcutaneous vasculature tissue and tenting of skin may be evident
-
Glucocorticoid excess may cause increased lanugo facial hair
-
Hirsutism and male pattern balding may be present in women if glucocorticoid excess is accompanied by androgen excess, as occurs in adrenocortical carcinomas
-
Steroid acne, consisting of papular or pustular lesions over the face, chest, and back, may be present
-
Acanthosis nigricans, which is associated with insulin resistance and hyperinsulinism, may be present
Gastroenterologic and skeletal/muscular signs and symptoms can include the following:
-
Peptic ulceration may occur with or without symptoms
-
Proximal muscle weakness may be evident
-
Osteoporosis may lead to incident fractures and kyphosis, height loss, and axial skeletal bone pain
-
Avascular necrosis of the hip is possible from glucocorticoid excess
Physical findings that occur in a patient in adrenal crisis include hypotension, abdominal pain, vomiting, and mental confusion (secondary to low serum sodium or hypotension). Other findings include hypoglycemia, hyperkalemia, hyponatremia, and metabolic acidosis.
Workup in Cushing syndrome
As mentioned, the diagnosis of Cushing syndrome requires demonstration of an inappropriately high level of cortisol in the serum or urine. The following tests have been recommended as screening tests for Cushing syndrome [2, 3] :
-
24-hour urine free cortisol
-
Low-dose dexamethasone suppression test (Liddle test)
An adrenocorticotropic hormone (ACTH) level obtained at the same time as the cortisol level can be helpful in identifying the etiology of Cushing syndrome.
Management of Cushing syndrome
The treatment for exogenous Cushing syndrome is gradual withdrawal of the causative drug, with the aim of discontinuing the causative drug if possible. An individual with hypothalamic-pituitary-adrenal (HPA)–axis suppression cannot increase steroid production appropriately during a medical illness or other stress and should receive stress-dose steroids to avoid adrenal crisis. [7]
Frequency
Most cases of Cushing syndrome are due to exogenous glucocorticoids. Prevalence of exogenous Cushing syndrome depends on the frequency and spectrum of medical conditions requiring glucocorticoid treatment in a given population. Considerable variation in this frequency is observed in populations of different cultural and ethnic backgrounds.
Mortality/Morbidity
Morbidity and mortality associated with Cushing syndrome are related primarily to the effects of excess glucocorticoids.
Two catastrophic medical crises that occur in glucocorticoid excess states are perforated viscera and opportunistic fungal infections. Exposure to excess glucocorticoids results in multiple medical problems, including hypertension, obesity, osteoporosis, fractures, impaired immune function, impaired wound healing, glucose intolerance, and psychosis.
Exogenous steroids suppress the hypothalamic-pituitary-adrenal (HPA) axis, with full recovery taking as long as a year after cessation of glucocorticoid administration. Thus, patients who are on or who have taken steroids are at risk for developing an adrenal crisis if steroids are stopped or not increased during an acute illness.
Pathophysiology
Glucocorticoids’ bioavailability is between 60% and 100%. More than 90% of the circulating glucocorticoid binds to corticosteroid binding globulin (CBG).The unbound free hormone in the circulation binds to the glucocorticoid receptor (GR). GR consists of a carboxy terminal ligand binding domain, a DNA binding domain and an N terminal domain. Except for prednisolone, which has an affinity for CBG that is about half of cortisol. Other synthetic glucocorticoids, in comparison to cortisol, have much less affinity to CBG.
Binding of the glucocorticoid to GR results in several intracellular processes of gene transcription and translation that ultimately lead to several actions of glucocorticoids on tissues. Some glucocorticoids can have cross activity with mineralocorticoid receptor (MR) due to significant homology between GR and MR. [8]
Structural differences between glucocorticoid compounds result in different bioavailability, duration, onset of action, potency and metabolic profiles of each product. Downregulation of the nuclear factor-kappa B activation, [9] changes in the enzyme adenosine monophosphate-activated protein kinase activity, [10] and modulation of activator protein 1 (Fos/Jun) [11] are some of the important pathways that have been described. More research still needs to be conducted to fully understand the underlying signaling pathways and glucocorticoid tissue-specific responses.
A study by Serfling et al suggested that weight gain in iatrogenic Cushing syndrome may be related to a glucocorticoid-stimulated rise in the amygdala and insula’s blood oxygen level–dependent (BOLD) response to approach-associated food stimuli. Thus, glucocorticoids may increase the anticipated reward value of food, leading to greater food consumption. [12]
Table 1. Glucocorticoid Equivalencies [13] (Open Table in a new window)
Type |
Drug |
Dose |
Relative Glucocorticoid Potency |
Relative Mineralocorticoid Potency |
Plasma Half-Life (mg) |
Biologic Half-Life (h) |
Short-acting |
Cortisol |
20 |
1.0 |
2 |
90 |
8-12 |
Hydrocortisone‡ |
25 |
0.8 |
2 |
80-118 |
8-12 |
|
Intermediate-acting |
Prednisone |
5 |
4 |
1 |
60 |
18-36 |
Prednisolone |
5 |
4 |
1 |
115-200 |
18-36 |
|
Triamcinolone |
4 |
5 |
0 |
30 |
18-36 |
|
Methylprednisolone |
4 |
5 |
0 |
180 |
18-36 |
|
Long-acting |
Dexamethasone |
0.5 |
25-50 |
0 |
200 |
36-54 |
Betamethasone |
0.6 |
25-50 |
0 |
300 |
36-54 |
|
Mineralocorticoid |
Aldosterone |
0.3 |
0 |
300 |
15-20 |
8-12 |
Fludrocortisone |
2 |
15 |
150 |
200 |
18-36 |
|
Desoxycorticosterone acetate |
0 |
0 |
20 |
70 |
… |
-
Physical findings in Cushing syndrome.
-
Diagnosis of Cushing syndrome.